665290
(S,S,S)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a′]dinaphthalen-4-yl)bis(1-phenylethyl)amine
97%
Sinonimo/i:
(+)-N,N-Bis[(1S)-1-phenylethyl]- dinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin- 4-amine, (11bR)
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Forma fisica
solid
Punto di fusione
88-89 °C
Gruppo funzionale
amine
phenyl
Stringa SMILE
C[C@H](N([C@@H](C)c1ccccc1)P2Oc3ccc4ccccc4c3-c5c(O2)ccc6ccccc56)c7ccccc7
InChI
1S/C36H30NO2P/c1-25(27-13-5-3-6-14-27)37(26(2)28-15-7-4-8-16-28)40-38-33-23-21-29-17-9-11-19-31(29)35(33)36-32-20-12-10-18-30(32)22-24-34(36)39-40/h3-26H,1-2H3/t25-,26-/m0/s1
LKZPDRCMCSBQFN-UIOOFZCWSA-N
Categorie correlate
Applicazioni
- Iridium-catalyzed allylic etherification of acyclic, achiral allylic carbonates with potassium silanolates to form chiral allylic alcohols.
- Palladium-catalyzed asymmetric allylic cyclisation of N-tosyl and N-benzyl carbonates to form the corresponding pyrrolidine and piperidine derivatives, respectively.
- Intramolecular iridium-catalyzed allylic cyclizationof (E)-allylic methyl carbonates to form 2,5-trans/cis pyrrolidine derivatives.
Caratteristiche e vantaggi
- Superior enantiocontrol in numerous transformations
- High activities at low catalyst loadings
- Hydrogenations under low-pressure conditions
Note legali
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
A diverse array of these chiral, monodentate phosphoramidites based on the privileged BINOL platform. The MonoPhos™ family has exhibited high levels of enantiocontrol in synthetic transformations ranging from metal-catalyzed asymmetric 1,4-additions of organometallic reagents to allylic alkylations to desymmetrization of meso-cycloalkene oxides.
In collaboration with DSM, we are pleased to offer a range of MonoPhos™ ligands for the research market.† Feringa and co-workers have invented a diverse array of these chiral, monodentate phosphoramidites based on the privileged BINOL platform.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.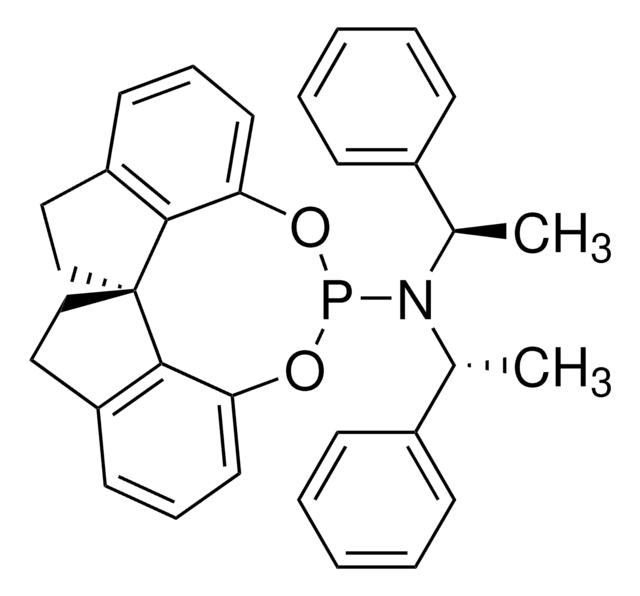
![(S,R,R)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a′]dinaphthalen-4-yl)bis(1-phenylethyl)amine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/366/790/7555ef31-5d0b-45c9-ad40-5dfd0fe85125/640/7555ef31-5d0b-45c9-ad40-5dfd0fe85125.png)
![(S)-(+)-N-(3,5-Dioxa-4-phosphacyclohepta[2,1-a;3,4-a′]dinaphthalen-4-yl)-dibenzo[b,f]azepine ≥95% (elemental analysis)](/deepweb/assets/sigmaaldrich/product/structures/575/489/d54360f9-5a59-43f2-bc44-42f5fa92b588/640/d54360f9-5a59-43f2-bc44-42f5fa92b588.png)
![(S)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a;3,4- a′]dinaphthalen-4-yl)dimethylamine 97%](/deepweb/assets/sigmaaldrich/product/structures/400/008/628143de-3954-440a-ba9c-4c0ff8e44663/640/628143de-3954-440a-ba9c-4c0ff8e44663.png)
![1,8-diazabiciclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane 98%](/deepweb/assets/sigmaaldrich/product/structures/189/812/8a6555e5-8de6-4236-865f-19339cee3634/640/8a6555e5-8de6-4236-865f-19339cee3634.png)

![(S,R)-(+)-(3,5-Dioxa-4-phospha-cyclohepta[2,1-a;4-a′]dinaphthalen-4-yl)-(1-phenylethyl)amine 96%](/deepweb/assets/sigmaaldrich/product/structures/340/157/5071e653-a834-4559-9aa7-4eb2d3774e42/640/5071e653-a834-4559-9aa7-4eb2d3774e42.png)
![(−)-Bis[(S)-1-phenylethyl]amine 99%](/deepweb/assets/sigmaaldrich/product/structures/336/455/d6f04f0e-9bcc-4d67-a94d-d153e39209e1/640/d6f04f0e-9bcc-4d67-a94d-d153e39209e1.png)