539112
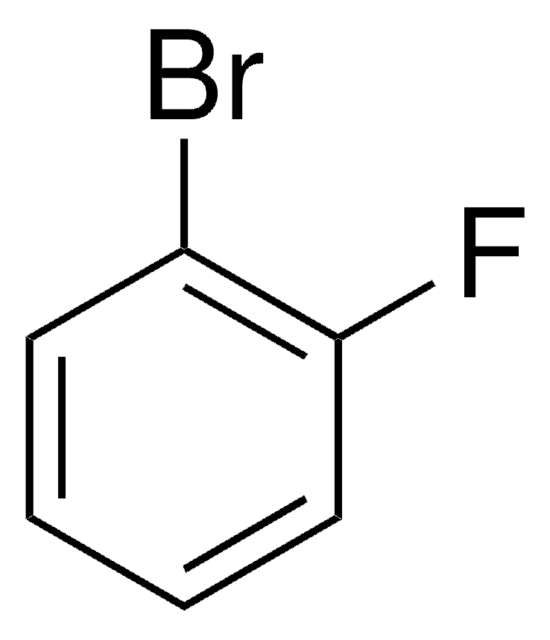
4-Bromo-1-fluoro-2-nitrobenzene
96%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
BrC6H3(F)NO2
Numero CAS:
Peso molecolare:
220.00
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
96%
Indice di rifrazione
n20/D 1.575 (lit.)
P. ebollizione
240-241 °C (lit.)
Punto di fusione
18-19 °C (lit.)
Densità
1.786 g/mL at 25 °C (lit.)
Gruppo funzionale
bromo
fluoro
nitro
Stringa SMILE
[O-][N+](=O)c1cc(Br)ccc1F
InChI
1S/C6H3BrFNO2/c7-4-1-2-5(8)6(3-4)9(10)11/h1-3H
UQEANKGXXSENNF-UHFFFAOYSA-N
Descrizione generale
4-Bromo-1-fluoro-2-nitrobenzene undergoes Sonogashira reaction with 2-fluoronitrobenzene to afford predominantly the bromo displacement product.
Applicazioni
4-Bromo-1-fluoro-2-nitrobenzene may be used in the synthesis of:
- 6-bromo-1H-benzo[d][1,2,3]triazol-1-ol
- 2-(4-bromo-2-nitrophenylamino)-5-methylthiophene-3-carbonitrile
- dibenzoxazepine analog, as potent sodium channel blocker
- 4-(4-bromo-2-nitrophenyl)piperazine-1-carboxylic acid tert-butylester
Used in the synthesis of anti-inflammatory agents.
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Katie M Lutker et al.
Crystal growth & design, 8(1), 136-139 (2008-01-01)
Bis(5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrilyl)acetylene, a derivative of the highly polymorphic compound 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile (ROY) that possesses two chromophores electronically coupled through a triple bond, was found to be trimorphic. Structural data for two of these forms indicates that symmetry is maintained in one structure
Patrick L DeRoy et al.
Organic letters, 9(14), 2741-2743 (2007-06-08)
The nucleophilic aromatic substitution reaction between electron-deficient aryl fluorides and terminal alkynes is shown to be efficiently promoted by sodium bis(trimethylsilyl)amide as a base. Moderate to excellent yields of 2-ethynylnitrobenzene products can be obtained under mild conditions.
Erik Rytter Ottosen et al.
Journal of medicinal chemistry, 46(26), 5651-5662 (2003-12-12)
We wish to report the synthesis and structure-activity relationship (SAR) of a series of 4-aminobenzophenones, as a novel compound class with high antiinflammatory activity. Our initial lead, (4-[(2-aminophenyl)amino]phenyl)(phenyl)methanone (3), was systematically optimized and resulted in compounds that potently inhibited the
Stephen M Lynch et al.
Bioorganic & medicinal chemistry letters, 25(1), 43-47 (2014-12-04)
We have identified two related series of dibenzazepine and dibenzoxazepine sodium channel blockers, which showed good potency on Nav1.7 in FLIPR-based and electrophysiological functional assays.
Tomoki Kawai et al.
Nuclear medicine and biology, 40(5), 705-709 (2013-05-28)
As a first trial for in vivo imaging of β-secretase (BACE1) in Alzheimer's disease brain, we applied a novel non-peptidergic small molecule which has high affinity to the enzyme, naphthalene-1-carboxylic acid (3'-chloro-4'-fluoro-4-piperazin-1-yl-biphenyl-3-yl)amide (NCFB) into positron emission tomography (PET) probe. In
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)