525057
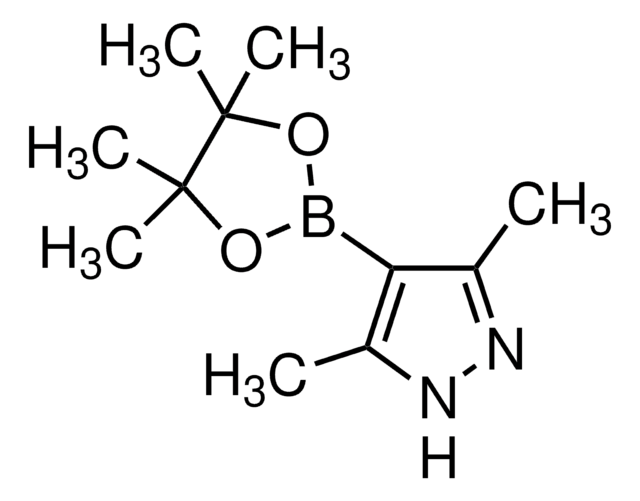
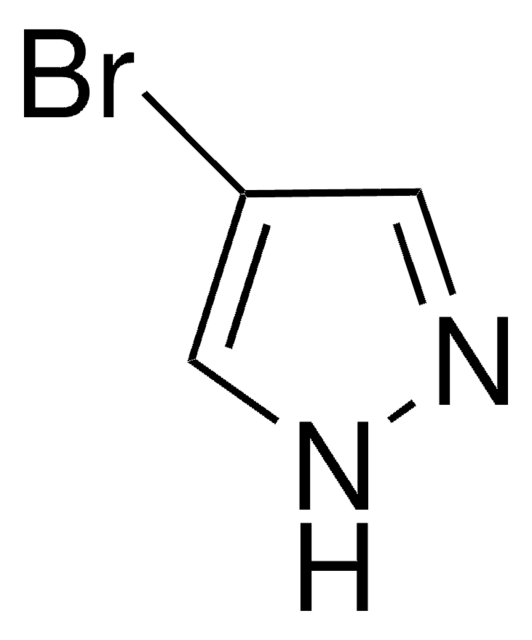
4-Pyrazoleboronic acid pinacol ester
97%
Sinonimo/i:
4,4,5,5-Tetramethyl-2-(1H-pyrazol-2-yl)-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-(1H-pyrazol-4-yl)-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-(pyrazol-4-yl)-1,3,2-dioxaborolane, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyrazole, Pyrazol-4-ylboronic acid pinacol ester
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
solid
Punto di fusione
142-146 °C (lit.)
Stringa SMILE
CC1(C)OB(OC1(C)C)c2cn[nH]c2
InChI
1S/C9H15BN2O2/c1-8(2)9(3,4)14-10(13-8)7-5-11-12-6-7/h5-6H,1-4H3,(H,11,12)
TVOJIBGZFYMWDT-UHFFFAOYSA-N
Categorie correlate
Applicazioni
- Suzuki-Miyaura cross-couplings
- Ruthenium-catalyzed asymmetric hydrogenation
Reagent used in preparation of inhibitors of many highly significant therapeutic enzymes and kinases containing the privileged scaffold pyrazole, including
- VEGF
- Aurora
- Rho (ROCK)
- Janus Kinase 2 (JAK)
- c-MET
- ALK
- S-nitrosoglutathione reductase
- CDC7
- Acetyl-CoA carboxylase
- Prosurvival Bcl-2 protein
- Viral RNA-Dependent RNA polymerase
- Long Chain Fatty Acid Elongase 6
- PI3
- AKT
- Chk1
- Protein Kinase B
Note legali
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 525057-5G | 4061833355718 |
| 525057-1G | 4061826696552 |
| 525057-25G | 4061833398784 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)