521418
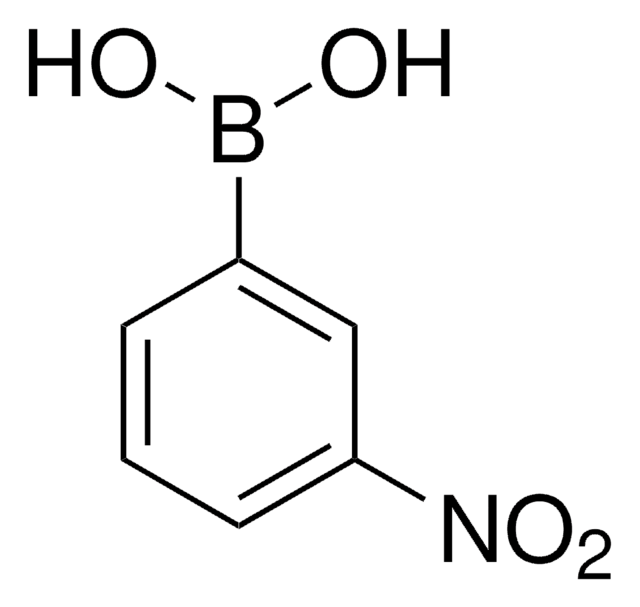
4-Cyanophenylboronic acid
≥95%
Sinonimo/i:
(p-Cyanophenyl)boronic acid, 4-Cyanobenzeneboronic acid, 4-Cyanophenylboric acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
NCC6H4B(OH)2
Numero CAS:
Peso molecolare:
146.94
Numero MDL:
Codice UNSPSC:
12352103
ID PubChem:
NACRES:
NA.22
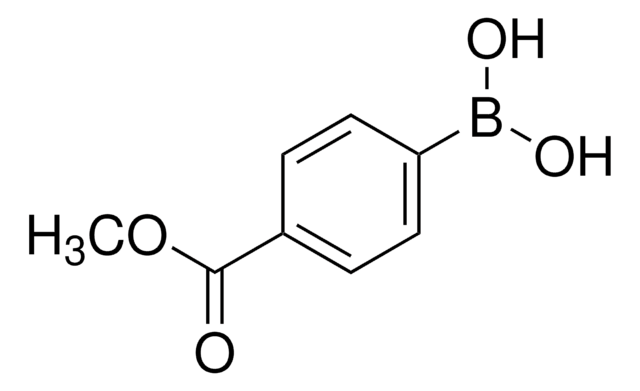
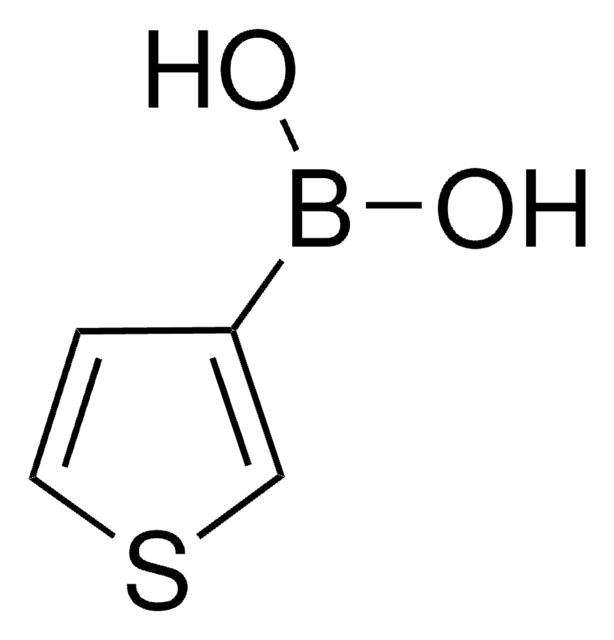
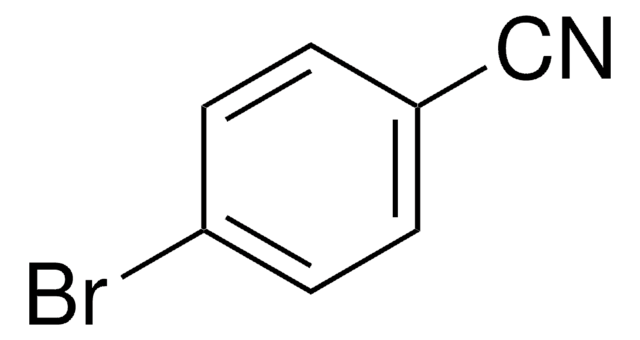
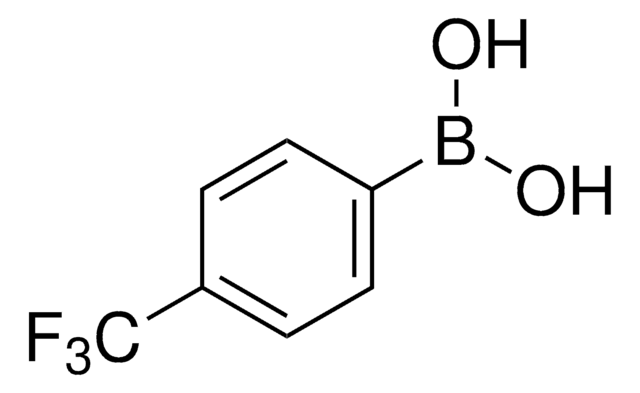
Prodotti consigliati
Livello qualitativo
Saggio
≥95%
Punto di fusione
>350 °C (lit.)
Gruppo funzionale
nitrile
Stringa SMILE
OB(O)c1ccc(cc1)C#N
InChI
1S/C7H6BNO2/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4,10-11H
CEBAHYWORUOILU-UHFFFAOYSA-N
Categorie correlate
Applicazioni
4-Cyanophenylboronic acid can be used as a reactant in:
It can also be used to prepare:
- Palladium-catalyzed Suzuki-Miyaura cross-coupling in water.
- Ruthenium catalyzed direct arylation of benzylic sp3 carbons of acyclic amines with arylboronates.
- Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.
- Ferric perchlorate-promoted reaction of fullerenes with various arylboronic acids to give fullerenyl boronic esters.
- Phosphine-free Suzuki-Miyaura cross-coupling.
- Palladacycles as effective catalysts for multicomponent reaction with allylpalladium-intermediates.
- Chan-Lam-type Cu-catalyzed S-arylation of thiols.
- Regioselective cross-coupling reactions under modfied Suzuki and Still cross-coupling reactions with copper catalysis.
- Metal-free biaryl coupling reaction in the presence of dimethyl carbonate as a solvent.
- Suzuki-type cross-coupling reaction with pentavalent triarylantimony diacetates in the absence of a base.
It can also be used to prepare:
- Himbacine analogs as thrombin receptor antagonists and potential antiplatelet agents.
- Trisulfonated calixarene upper-rim sulfonamido and their complexation with trimethyllysine epigenetic mark.
- Antimalarial compounds via Suzuki cross-coupling.
- Deoxyuridine derivatives.
Reactant involved in:
Precursor in the synthesis of inhibitors such as:
- Oxidative hydroxylation
- Trifluoromethylation
- 1,4-Addition reactions
Precursor in the synthesis of inhibitors such as:
- Tpl2 kinase inhibitors
- P2X7 antagonists used in the treatment of pain
Altre note
Contains varying amounts of anhydride
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Discovery of nor-seco himbacine analogs as thrombin receptor antagonists
Chelliah, M. V.; et al.
Bioorganic & Medicinal Chemistry, 22, 2544-2549 (2012)
Mohamed A Ismail et al.
Journal of medicinal chemistry, 47(14), 3658-3664 (2004-06-25)
2-[5-(4-Amidinophenyl)-furan-2-yl]-5,6,7,8-tetrahydro-imidazo[1,2-a]pyridine-6-carboxamidine acetate salt (7) was synthesized from 2-[5-(4-cyanophenyl)-furan-2-yl]-imidazo[1,2-a]pyridine-6-carbonitrile (4a), through the bis-O-acetoxyamidoxime followed by hydrogenation in glacial acetic acid. Compound 4a was obtained in four steps starting with two successive brominations of 2-acetylfuran first with N-bromosuccinimide, and second with bromine
A mild and efficient new synthesis of aryl sulfones from boronic acids and sulfinic acid salts
Christian Beaulieu, et al.
Tetrahedron Letters, 45, 3233-3236 (2004)
Palladacycles: Effective catalysts for a multicomponent reaction with allylpalladium(II)-intermediates
Shiota, A.; Malinakova, H. C.
Journal of Organometallic Chemistry, 704, 9-16 (2012)
Yassir Younis et al.
Journal of medicinal chemistry, 55(7), 3479-3487 (2012-03-07)
A novel class of orally active antimalarial 3,5-diaryl-2-aminopyridines has been identified from phenotypic whole cell high-throughput screening of a commercially available SoftFocus kinase library. The compounds were evaluated in vitro for their antiplasmodial activity against K1 (chloroquine and drug-resistant strain)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 521418-1G | 4061832547312 |
| 521418-10G | 4061832905150 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)