480061
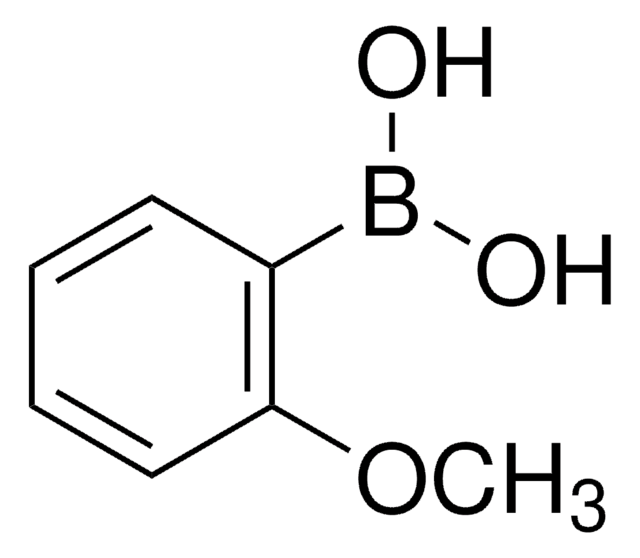
2,6-Dimethylphenylboronic acid
≥95.0%
Sinonimo/i:
2,6-Dimethylbenzeneboronic acid, 2,6-Xyleneboronic acid, 2,6-Xylylboronic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(CH3)2C6H3B(OH)2
Numero CAS:
Peso molecolare:
149.98
Numero MDL:
Codice UNSPSC:
12352103
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
≥95.0%
Impurezze
<10% water
Punto di fusione
105 °C (dec.) (lit.)
Stringa SMILE
Cc1cccc(C)c1B(O)O
InChI
1S/C8H11BO2/c1-6-4-3-5-7(2)8(6)9(10)11/h3-5,10-11H,1-2H3
ZXDTWWZIHJEZOG-UHFFFAOYSA-N
Categorie correlate
Applicazioni
Reagent used for
Reagent used in Prepration of
- Palladium catalyzed Suzuki-Miyaura coupling reactions
- One-pot ipso-nitration of arylboronic acids including broader substrate scope of heterocycles and functional groups
- Nickel-Catalyzed Cross-Coupling of Chromene Acetals and Boronic Acids
- Visible-light initiated aerobic oxidative hydroxylation catalyzed by Ru-complex
- Rhodium(I)-catalyzed 1,4-addition reactions
- Pd-catalyzed homocouplings
- Expanded scope of Cu assisted Suzuki-Miyaura coupling reactions including aryl chlorides and polyhalo aryl boronates
Reagent used in Prepration of
- Orally bioavialable G Protein-Coupled Receptor 40 agonists for diabetes treatment
- Solid phase synthesis and antitumor structure-activity relationship of Smac triazoloprolines and biarylalanines tetrapeptide libraries
- Protein Kinase inhibitors
Altre note
contains varying amounts of anhydride
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Room-temperature synthesis of tetra-ortho-substituted biaryls by NHC-catalyzed Suzuki-Miyaura couplings.
Linglin Wu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(46), 12886-12890 (2011-10-11)
Laura A T Cleghorn et al.
ChemMedChem, 6(12), 2214-2224 (2011-09-14)
New drugs are urgently needed for the treatment of tropical parasitic diseases such as leishmaniasis and human African trypanosomiasis (HAT). This work involved a high-throughput screen of a focussed kinase set of ~3400 compounds to identify potent and parasite-selective inhibitors
Expanding the scope of the Cu assisted Suzuki-Miyaura reaction
Crowley, B. M.; et al.
Tetrahedron Letters, 52, 5055-5059 (2011)
Satoshi Mikami et al.
Journal of medicinal chemistry, 55(8), 3756-3776 (2012-03-21)
As part of a program to identify potent GPR40 agonists with drug-like properties suitable for clinical development, the incorporation of polar substituents was explored with the intention of decreasing the lipophilicity of our recently disclosed phenylpropanoic acid derivative 1. This
Thomas J A Graham et al.
Organic letters, 14(6), 1616-1619 (2012-03-06)
A modular and highly efficient protocol for the synthesis of 2-aryl- and heteroaryl-2H-chromenes is described. Under base-free conditions, readily accessible 2-ethoxy-2H-chromenes undergo C(sp(3))-O activation and C(sp(3))-C bond formation in the presence of an inexpensive nickel catalyst and boronic acids. This
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.



![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)