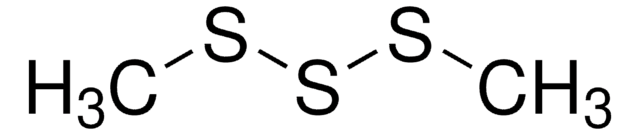471569
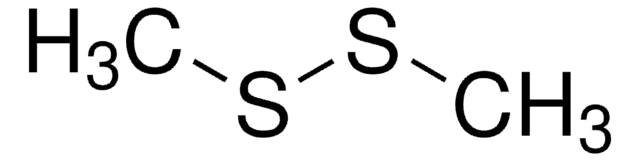
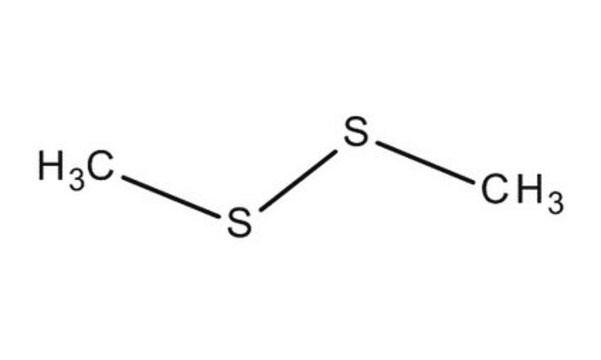
Dimethyl disulfide
≥99.0%
Sinonimo/i:
DMDS, Methyl disulfide
About This Item
Prodotti consigliati
Densità del vapore
3.24 (vs air)
Livello qualitativo
Tensione di vapore
22 mmHg ( 20 °C)
Saggio
≥99.0%
Temp. autoaccensione
>572 °F
Limite di esplosione
16 %
Indice di rifrazione
n20/D 1.525 (lit.)
P. ebollizione
109 °C (lit.)
Punto di fusione
−85 °C (lit.)
Densità
1.046 g/mL at 25 °C (lit.)
Gruppo funzionale
disulfide
Stringa SMILE
CSSC
InChI
1S/C2H6S2/c1-3-4-2/h1-2H3
WQOXQRCZOLPYPM-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Sens. 1 - STOT SE 1 Inhalation - STOT SE 3
Organi bersaglio
Central nervous system, Upper respiratory tract
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
59.0 °F - closed cup
Punto d’infiammabilità (°C)
15 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
as thiolate at the bridge site
Protocolli
Separation of 4-Methyl-2-pentanone; Dimethyl disulfide; Hexanal; 3-Methylpentane; Acetone
Separation of Sulfur dioxide; Hydrogen sulfide; Carbonyl sulfide; Methanethiol; Ethanethiol; Dimethyl disulfide; Carbon disulfide
Separation of 2-Ethyl-3-methylpyrazine; 1-Methylpyrrole; 2,3-Dimethylpyrazine; 2,5-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Diethylpyrazine; 2-Methylpyrazine; Carbon disulfide; Dimethyl disulfide; 2,6-Dimethylpyrazine
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.