441236
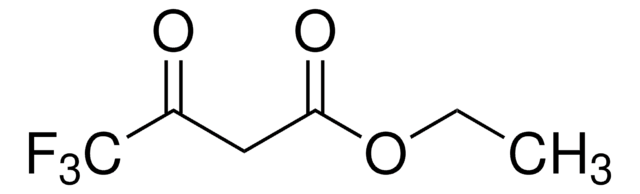
Ethyl 2-(ethoxymethylene)-4,4,4-trifluoro-3-oxobutyrate,mixture of cis and trans
96%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
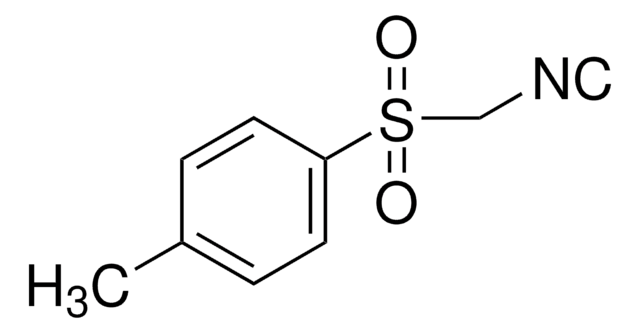
Formula condensata:
CF3COC(=CHOC2H5)CO2C2H5
Numero CAS:
Peso molecolare:
240.18
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
96%
Forma fisica
liquid
Indice di rifrazione
n20/D 1.429 (lit.)
P. eboll.
80-82 °C/1 mmHg (lit.)
Densità
1.235 g/mL at 25 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
CCO\C=C(\C(=O)OCC)C(=O)C(F)(F)F
InChI
1S/C9H11F3O4/c1-3-15-5-6(8(14)16-4-2)7(13)9(10,11)12/h5H,3-4H2,1-2H3/b6-5+
XNGGOXOLHQANRB-AATRIKPKSA-N
Descrizione generale
Ethyl 2-(ethoxymethylene)-4,4,4-trifluoro-3-oxobutyrate has been reported to participate in the microwave-assisted synthesis of ethyl 1-[4-(2,3,3-trichloroacrylamido)phenyl]-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate.
Applicazioni
Ethyl 2-(ethoxymethylene)-4,4,4-trifluoro-3-oxobutyrate may be employed as a starting reagent for the synthesis of 1-methyl-3-trifluoromethyl-1H-pyrazole-4- carboxylic acid.
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
219.2 °F - closed cup
Punto d’infiammabilità (°C)
104.00 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
L Sansebastiano et al.
Farmaco (Societa chimica italiana : 1989), 48(3), 335-355 (1993-03-01)
The synthesis of ethyl or methyl 4-substituted or unsubstituted 2-methylthio-5-pyrimidinecarboxylates 3 a-i and 8 o mainly by reaction of ethyl or methyl 2-dimethylaminomethylene-3-oxoalkanoates with 2-methylisothiourea is described. Also some ethyl 2-substituted (NH2, CH3, C6H5) 4-trifluoromethyl-5-pyrimidinecarboxylates were prepared. Some of the
P J Sanfilippo et al.
Journal of medicinal chemistry, 38(1), 34-41 (1995-01-06)
The synthesis and biological activity of novel thiazole-based heterocycles as inhibitors of thrombin-induced human platelet aggregation are described. Further evaluation of selected compounds show they inhibit platelet aggregation as stimulated by a variety of agonists. The more active compounds also
R D Franz
AAPS pharmSci, 3(2), E10-E10 (2001-12-14)
The changes in the physiochemical properties accompanying the substitution of a phosphonic acid group for a carboxylic acid group on various heterocyclic platforms was determined. A series of low molecular weight heterocyclic carboxylic and phosphonic acids was prepared, and the
L Mosti et al.
Farmaco (Societa chimica italiana : 1989), 47(4), 427-437 (1992-04-01)
The synthesis of ethyl or methyl esters of 5-cyano-1,6-dihydro-6-oxo-3- pyridinecarboxylic acids carrying as 2-substituent a polar group such as CO2C2H5, (CH2)2CO2CH3, (CH2)3CO2C2H5, CH2OCH3, or CF3 group is described. Also 2-[5-cyano-1,6-dihydro-2-(1,1-dimethylethyl)-6-oxo-3-pyridyl]-2- oxoacetic acid and 2,5,6,8-tetrahydro-2,5-dioxo-1H-thiopyrano[3,4-b]pyridine-3-carbon itrile were prepared. Nearly all the
European Journal of Medicinal Chemistry, 28, 853-853 (1993)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.