433063
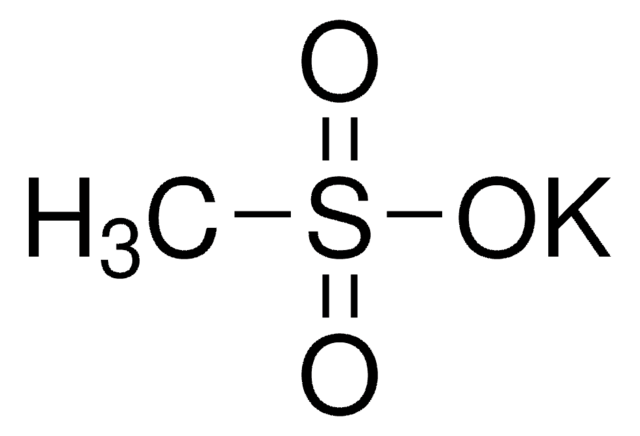
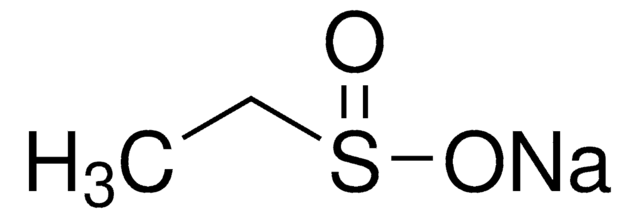
Sodium methanesulfinate
technical grade, 85%
Sinonimo/i:
Methanesulfinic acid sodium salt
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3SO2Na
Numero CAS:
Peso molecolare:
102.09
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Grado
technical grade
Livello qualitativo
Saggio
85%
Punto di fusione
222-226 °C (dec.) (lit.)
Gruppo funzionale
sulfinic acid
Stringa SMILE
[Na+].CS([O-])=O
InChI
1S/CH4O2S.Na/c1-4(2)3;/h1H3,(H,2,3);/q;+1/p-1
LYPGDCWPTHTUDO-UHFFFAOYSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Sodium methanesulfinate is an aliphatic sodium sulfinate. Conjugate addition of sodium methanesulfinate to vinyl heterocycles has been described. Cross-coupling reaction between aryl boronic acid and sodium methanesulfinate has been studied. Its stock solution was prepared from methanesulfonic acid by adding one equivalent of sodium hydroxide and diluting it to 4M.
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Metal-Free Direct Construction of Sulfonamides via Iodine-Mediated Coupling Reaction of Sodium Sulfinates and Amines at Room Temperature.
Wei W, et al.
Advanced Synthesis & Catalysis, 357(5), 987-992 (2015)
J B Smith et al.
Free radical research communications, 8(2), 101-106 (1990-01-01)
Iron bound to certain chelators is known to promote the conversion of superoxide radicals (O2-) to hydroxyl radicals (HO.) by the superoxide-driven Fenton reaction. The production of HO. by various iron chelates was studied using the reaction of dimethyl sulfoxide
M G Steiner et al.
Archives of biochemistry and biophysics, 278(2), 478-481 (1990-05-01)
This investigation was conducted to validate the use of dimethyl sulfoxide (DMSO) as a quantitative molecular probe for the generation of hydroxyl radicals (HO.) in aqueous systems. Reaction of HO. with DMSO produces methane sulfinic acid as a primary product
M G Steiner et al.
Free radical biology & medicine, 9(1), 67-77 (1990-01-01)
To quantitate the formation of hydroxyl radicals (HO.) in ischemia and reoxygenation, dimethyl sulfoxide (DMSO) was added to "trap" evolving HO. in normal, in ischemic, and in ischemic and reoxygenated rat kidney slices, incubated in short-term organ culture in vitro.
S Fukui et al.
Journal of chromatography, 630(1-2), 187-193 (1993-02-05)
For the determination of hydroxyl radicals, dimethyl sulphoxide was used as a molecular probe and the methanesulphinic acid produced was determined by high-performance liquid chromatography of its Fast Yellow GC salt derivative. The results for hydroxyl radicals formed using the
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![1,8-diazabiciclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)