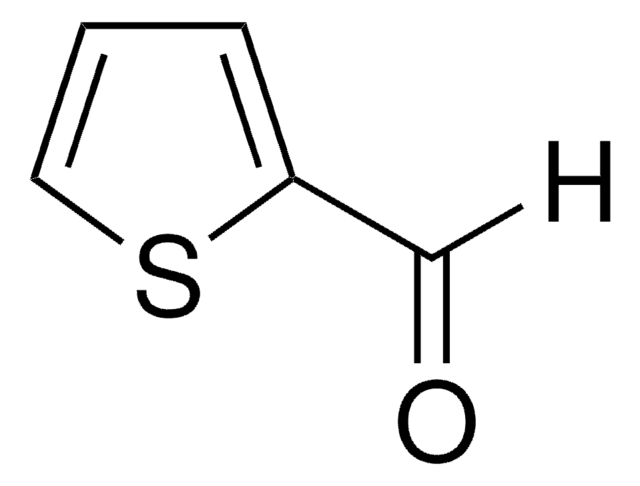
429880
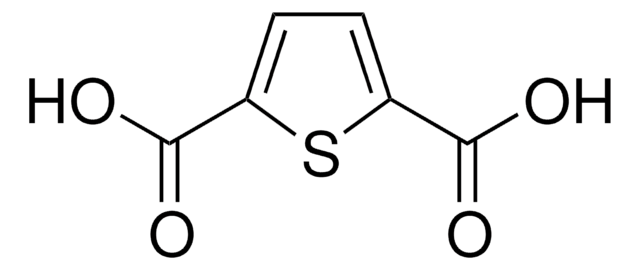
2,5-Thiophenedicarboxaldehyde
99%
Sinonimo/i:
2,5-Diformylthiophene, 2,5-Thienodicarboxaldehyde, 2,5-Thiophenedial, Thiophene-2,5-dialdehyde
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H4O2S
Numero CAS:
Peso molecolare:
140.16
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Punto di fusione
115-117 °C (lit.)
Gruppo funzionale
aldehyde
Stringa SMILE
[H]C(=O)c1ccc(s1)C([H])=O
InChI
1S/C6H4O2S/c7-3-5-1-2-6(4-8)9-5/h1-4H
OTMRXENQDSQACG-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
2,5-Thiophenedicarboxaldehyde can be prepared from 2,5-bis(chloromethyl)thiophene by the application of Kröhnke′s method.
Applicazioni
2,5-Thiophenedicarboxaldehyde may be employed in the following studies:
- Asymmetric synthesis of bis-homoallylic alcohols.
- Synthesis of new symmetrical arylene bisimide derivatives.
- As dialdehyde monomer in the synthesis of silicon-containing poly(p-phenylenevinylene)-related copolymers having uniform p-conjugated segment regulated by organosilicon units.
- Synthesis of 2,5-bis[2-(5-N-isopropylamidino)benzimidazoyl]thiophene hydrochloride.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Xiaoming Li et al.
Frontiers in oncology, 8, 354-354 (2018-10-16)
RNA interference (RNAi) is a biological process through which gene expression can be inhibited by RNA molecules with high selectivity and specificity, providing a promising tool for tumor treatment. Two types of molecules are often applied to inactivate target gene
M Del Poeta et al.
Antimicrobial agents and chemotherapy, 42(10), 2495-2502 (1998-10-03)
Twenty analogues of pentamidine, 7 primary metabolites of pentamidine, and 30 dicationic substituted bis-benzimidazoles were screened for their inhibitory and fungicidal activities against Candida albicans and Cryptococcus neoformans. A majority of the compounds had MICs at which 80% of the
The Preparation of 2, 5-Thiophenedicarboxaldehyde.
Sone T.
Bulletin of the Chemical Society of Japan, 37(8), 1197-1200 (1964)
Marzena Grucela-Zajac et al.
The journal of physical chemistry. C, Nanomaterials and interfaces, 118(24), 13070-13086 (2014-06-27)
New symmetrical arylene bisimide derivatives formed by using electron-donating-electron-accepting systems were synthesized. They consist of a phthalic diimide or naphthalenediimide core and imine linkages and are end-capped with thiophene, bithiophene, and (ethylenedioxy)thiophene units. Moreover, polymers were obtained from a new
Guang-Ming Chen et al.
The Journal of organic chemistry, 64(3), 721-725 (2001-10-25)
Asymmetric reduction of 2,6-diacylpyridines with B-chlorodiisopinocampheylborane provides the corresponding C(2)-symmetric diols in very high de and ee. Asymmetric allylboration of 2,6-pyridinedicarboxaldehyde and 2,5-thiophenedicarboxaldehyde provides the corresponding bis-homoallylic alcohols in very high de and ee. These optically pure diols were converted
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)