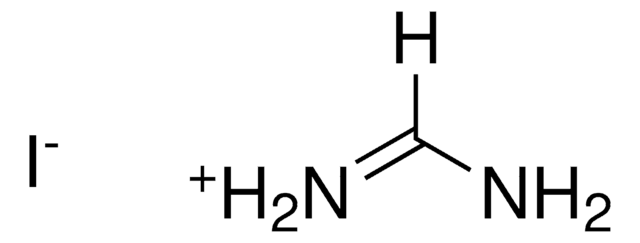
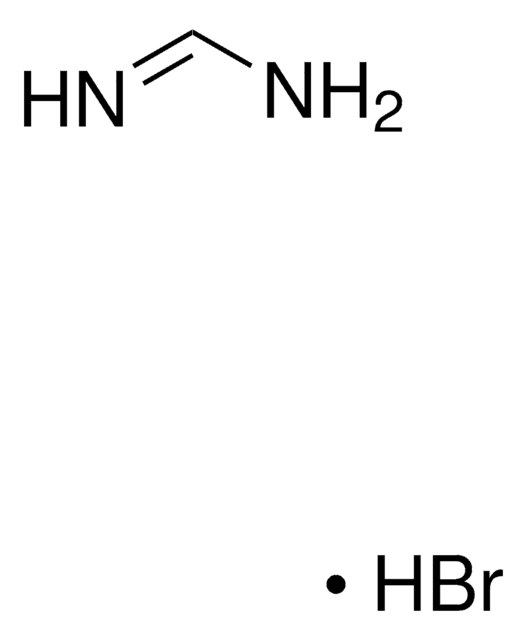
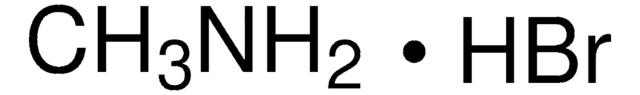398853
Lead(II) bromide
99.999% trace metals basis
Sinonimo/i:
Lead dibromide
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
99.999% trace metals basis
Stato
powder
Impiego in reazioni chimiche
core: lead
Impurezze
≤15.0 ppm Trace Metal Analysis
P. ebollizione
892 °C (lit.)
Punto di fusione
371 °C (lit.)
Densità
6.66 g/mL at 25 °C (lit.)
Stringa SMILE
Br[PbH2]Br
InChI
1S/2BrH.Pb/h2*1H;/q;;+2/p-2
ZASWJUOMEGBQCQ-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- To prepare an electrolyte for a high performance all-solid-state bromide-ion battery.
- As a precursor to prepare organic–inorganic hybrid perovskite materials for solar cells and light emitting devices (LEDs).
- As a starting material to prepare photocatalytic CsPbBr3@SiO2 composites with good water stability.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Repr. 1A - STOT RE 2
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Elenchi normativi
Forniamo informazioni su eventuali restrizioni prevalentemente per i prodotti chimici. Per altre tipologie di prodotto siamo in grado di fornire soltanto informazioni limitate. Nessuna segnalazione significa che nessuno dei componenti è citato in un elenco. È dovere dell’utilizzatore assicurarsi che il prodotto venga impiegato in maniera sicura e a norme di legge.
EU REACH Annex XVII (Restriction List)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Since the first report of the low-cost dye-sensitized solar cell (DSSC) in 1991 by Gratzel and his coworker,1 dye-sensitized solar cells (DSSC) has been regarded as one of the most promising photovoltaic technologies because of their transparent and colorful characteristics, as well as low cost.
Colloidal quantum dots (CQDs) are semiconducting crystals of only a few nanometers (ca. 2–12 nm) coated with ligand/surfactant molecules to help prevent agglomeration.
The past several decades have seen major advancements in the synthesis of metal nanomaterials. Most recently, controlled synthesis has become versatile enough to regulate the exact number of atoms and ligands of very small metal nanoparticles, referred to as “clusters”.
Dr. Perini and Professor Correa-Baena discuss the latest research and effort to obtain higher performance and stability of perovskite materials.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.