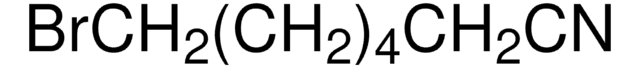392278
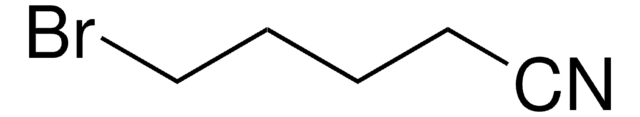
6-Bromohexanenitrile
95%
Sinonimo/i:
5-Bromopentyl cyanide, 6-Bromocapronitrile
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
Br(CH2)5CN
Numero CAS:
Peso molecolare:
176.05
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
95%
Stato
liquid
Indice di rifrazione
n20/D 1.477 (lit.)
P. ebollizione
134 °C/13 mmHg (lit.)
Densità
1.328 g/mL at 25 °C (lit.)
Gruppo funzionale
bromo
nitrile
Stringa SMILE
BrCCCCCC#N
InChI
1S/C6H10BrN/c7-5-3-1-2-4-6-8/h1-5H2
PHOSWLARCIBBJZ-UHFFFAOYSA-N
Descrizione generale
6-Bromohexanenitrile (6-Bromohexanonitrile) is an ω-bromoalkanonitrile. Friedel Crafts alkylation of 6-bromohexanenitrile with benzene has been studied.
Applicazioni
6-Bromohexanenitrile (6-Bromocapronitrile, 6-Bromohexanonitrile) is suitable reagent used in the synthesis of (5-cyanopentyl)zinc(II) bromide, an organozinc reagent. It may be used in the synthesis of the following:
- 6-(1,4-dioxa-8-azaspiro[4.5]dec-8-yl)hexanenitrile.
- dimethyl 6,6′-dithiobiscaproimidate, a long-chain dithiobisimidate.
- N-benzyloxy-(4-cyanopentyl)-carbamic acid ethyl ester, a N-benzyloxy carbamate derivative.
- 1-hetarylsulfanyl-ω-cyanoalkanes.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Friedel-Crafts alkylation of benzene by normal ?-chloroalkanoic acids and their methyl esters and nitriles.
Zakharkin LI and Anikina EV
Bulletin of the Academy of Sciences of the USSR, Division of chemical science, 36(2), 327-330 (1987)
H Peretz et al.
European journal of biochemistry, 63(1), 77-82 (1976-03-16)
This communication describes a simple method for synthesizing cleavable bifunctional imido esters of different chain lengths. These reagents, which form covalent crosslinks between lysine residues of proteins, contain a disulfide bond which is cleaved under mild conditions by reducing agents
?Green Chemical" Methods for the Regioselective Synthesis of 1-Hetarylsulfanyl-?-Cyanoalkanes."
Abele E, et al.
Latvijas Kimijas Zurnals, 49(1), 278-282 (2010)
NICKEL-CATALYZED ENANTIOSELECTIVE NEGISHI CROSS-COUPLINGS OF RACEMIC SECONDARY α-BROMO AMIDES WITH ALKYLZINC REAGENTS: (S)-N-BENZYL-7-CYANO-2-ETHYL-N-PHENYLHEPTANAMIDE.
Sha Lou et al.
Organic syntheses; an annual publication of satisfactory methods for the preparation of organic chemicals, 87, 330-330 (2010-01-01)
Yuan Liu et al.
Tetrahedron, 67(12), 2206-2214 (2011-04-19)
N-Alkyl-N-benzyloxy carbamates, 2, undergo facile intramolecular cyclization with a variety of carbon nucleophiles to give functionalized 5- and 6-membered protected cyclic hydroxamic acids, 3, in good to excellent yields. This method can be extended to prepare seven-membered cyclic hydroxamic acids
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.