391204
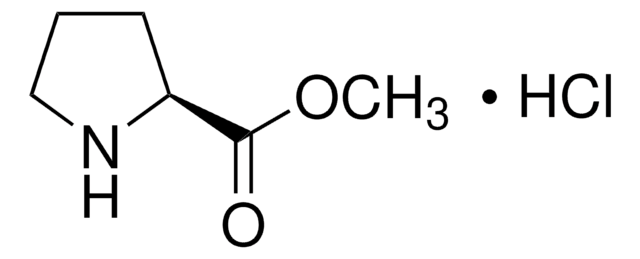
Methyl pipecolinate hydrochloride
97%
Sinonimo/i:
Methyl 2-piperidinecarboxylate hydrochloride
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C7H13NO2 · HCl
Numero CAS:
Peso molecolare:
179.64
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Punto di fusione
205 °C (dec.) (lit.)
Gruppo funzionale
ester
Stringa SMILE
Cl.COC(=O)C1CCCCN1
InChI
1S/C7H13NO2.ClH/c1-10-7(9)6-4-2-3-5-8-6;/h6,8H,2-5H2,1H3;1H
APCHKWZTSCBBJX-UHFFFAOYSA-N
Descrizione generale
Methyl pipecolinate hydrochloride is a hydrochloride salt of methyl piperidine-2-carboxylate (methyl pipecolinate). The kinetics of the enzymatic separation of enantiomeric forms of methyl pipecolinate using Candida antarctica Lipase A (CAL-A) has been reported. Its role as catalyst for the standard Diels-Alder reaction has been examined.
Applicazioni
Reactant for synthesis of:
A pipecolic linker
Antiviral agents
Aurora and epidermal growth factor receptor kinase inhibitor
Pyrrolidine derivatives via reduction of substituted pyrroles
Reactant for:
Petasis reactions
Decarbonylative radical cyclization of alpha-amino selenoesters upon electrophilic alkenes
A pipecolic linker
Antiviral agents
Aurora and epidermal growth factor receptor kinase inhibitor
Pyrrolidine derivatives via reduction of substituted pyrroles
Reactant for:
Petasis reactions
Decarbonylative radical cyclization of alpha-amino selenoesters upon electrophilic alkenes
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Advances in the kinetic and dynamic kinetic resolution of piperazine-2-carboxylic acid derivatives with Candida antarctica lipase A; structural requirements for enantioselective N-acylation.
Hietanen A, et al.
ARKIVOC (Gainesville, FL, United States), 5, 60-74 (2012)
Aldehyde-based racemization in the dynamic kinetic resolution of N-heterocyclic a-amino esters using Candida Antarctica lipase A.
Liljeblad A, et al.
Tetrahedron, 60(3), 671-677 (2004)
The α-effect in cyclic secondary amines: new scaffolds for iminium ion accelerated transformations.
Brazier JB, et al.
Tetrahedron, 65(48), 9961-9966 (2009)
Enantioselective lipase-catalyzed reactions of methyl pipecolinate: transesterification and N-acylation.
Liljeblad, A, et al.
Tetrahedron Letters, 43(13), 2471-2474 (2002)
Alkoxycarbonylpiperidines as N-nucleophiles in the palladium-catalyzed aminocarbonylation.
Takacs A, et al.
Monatshefte fur Chemie / Chemical Monthly, 145(9), 1473-1478 (2014)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.