377155
Chlorobis(cyclooctene)iridium(I)dimer
97%
Sinonimo/i:
[Ir(coe)2Cl]2, Di-μ-chlorotetrakis(cyclooctene)diiridium
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
powder
Impiego in reazioni chimiche
core: iridium
reagent type: catalyst
Punto di fusione
160-165 °C (dec.) (lit.)
Stringa SMILE
Cl[Ir].Cl[Ir].[CH]1[CH]CCCCCC1.[CH]2[CH]CCCCCC2.[CH]3[CH]CCCCCC3.[CH]4[CH]CCCCCC4
InChI
1S/4C8H14.2ClH.2Ir/c4*1-2-4-6-8-7-5-3-1;;;;/h4*1-2H,3-8H2;2*1H;;/q;;;;;;2*+1/p-2/b4*2-1-;;;;
WBRREXQCZAFSKS-XFCUKONHSA-L
Categorie correlate
Applicazioni
- Isomerization-hydroboration reactions with nido-carboranyldiphosphine as stabilizing ligand
- Hydrogen peroxide oxidation of hydroxamic acids and their subsequent hetero Diels-Alder cycloaddition reactions
- Immobilization of organic functional groups onto solid supports through vinylsilane coupling reactions
- Alkylation reactions
- Guerbet reaction
- Allylic amination reactions in a DNA-diene-iridium(I) hybrid system
- Asymmetric hydroamination reactions
- Isomerization-hydroboration reactions with nido-carboranyldiphosphine as stabilizing ligand.
- Hydrogen peroxide oxidation of hydroxamic acids and their subsequent hetero Diels-Alder cycloaddition reactions.
- Immobilization of organic functional groups onto solid supports through vinylsilane coupling reactions.
- Alkylation reactions.
- Guerbet reaction.
- Allylic amination reactions using a DNA-diene-iridium(I) hybrid system.
- Asymmetric hydroamination reactions.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Arylboronic acids and esters are invaluable tools for the chemical community. These powerful reagents are used for a variety of transformations, most notably the Suzuki-Miyaura cross-coupling reaction.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.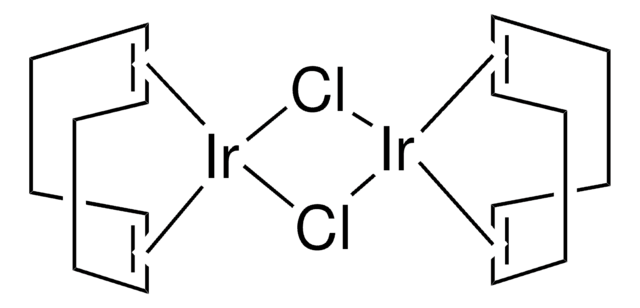





![[Ir(cod)(acac)] Umicore](/deepweb/assets/sigmaaldrich/product/structures/188/615/470bfca9-6b61-476a-9486-f7da61962e4c/640/470bfca9-6b61-476a-9486-f7da61962e4c.png)