234893
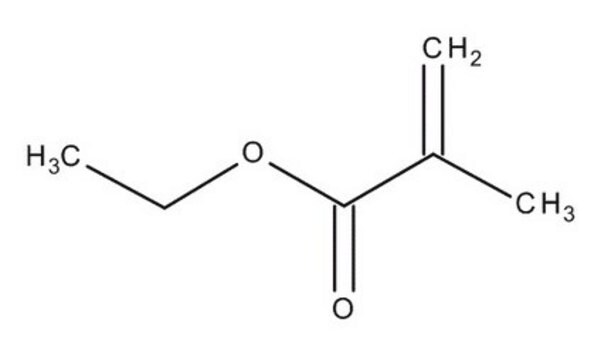
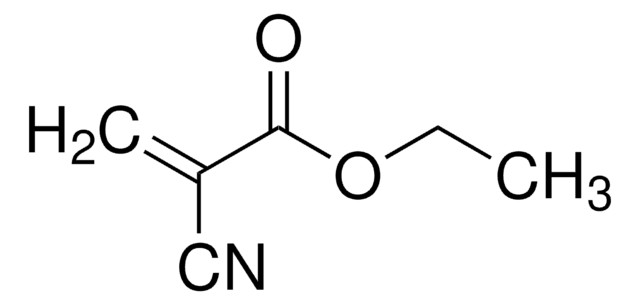
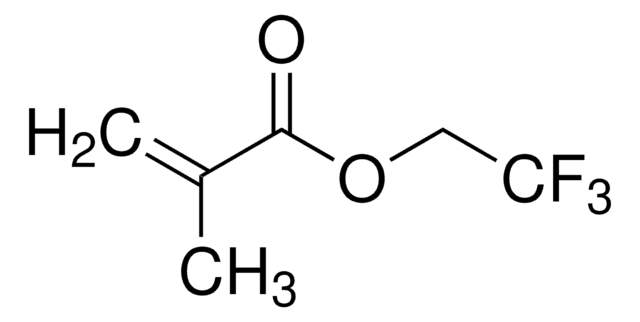
Ethyl methacrylate
contains 15-20 ppm monomethyl ether hydroquinone as inhibitor, 99%
Sinonimo/i:
2-Methyl-2-propenoic acid
About This Item
Prodotti consigliati
Densità del vapore
>3.9 (vs air)
Livello qualitativo
Tensione di vapore
15 mmHg ( 20 °C)
Saggio
99%
Forma fisica
liquid
Temp. autoaccensione
771 °F
contiene
15-20 ppm monomethyl ether hydroquinone as inhibitor
Indice di rifrazione
n20/D 1.413 (lit.)
P. eboll.
118-119 °C (lit.)
Densità
0.917 g/mL at 25 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
CCOC(=O)C(C)=C
InChI
1S/C6H10O2/c1-4-8-6(7)5(2)3/h2,4H2,1,3H3
SUPCQIBBMFXVTL-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Ethyl methacrylate is a readily polymerizable monomer used for certain types of acrylic resins. The monomethyl ether hydroxyl quinone present in it is an inhibitor that prevents polymerization.
Applicazioni
- To synthesize artificial nanosized latexes of poly(styrene-co-methyl methacrylate) or poly(styrene-co-ethyl methacrylate), which are in producing drug-releasing films.
- In the production of additive-manufactured methacrylate-based resins used in dentistry.
- In the synthesis of a star-shaped block copolymer electrolyte for all-solid-state lithium batteries.
- In the synthesis of a copolymer used as a matrix for semiconductor nanoparticles, which is crucial for the formation of a stable matrix for the quantum dots-copolymer composite material used in optoelectronic applications.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
66.2 °F - closed cup
Punto d’infiammabilità (°C)
19 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.



![2-[3-(2H-Benzotriazol-2-yl)-4-hydroxyphenyl]ethyl methacrylate 99%](/deepweb/assets/sigmaaldrich/product/structures/208/967/cf29567e-c125-41dc-b80a-66889fa1a679/640/cf29567e-c125-41dc-b80a-66889fa1a679.png)