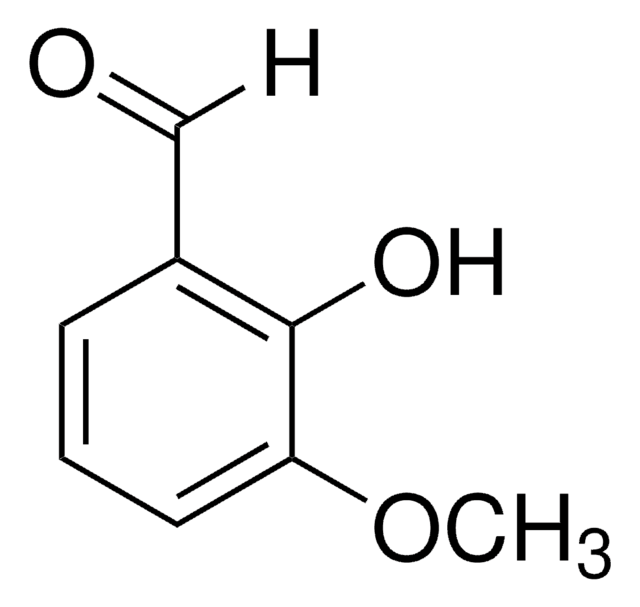
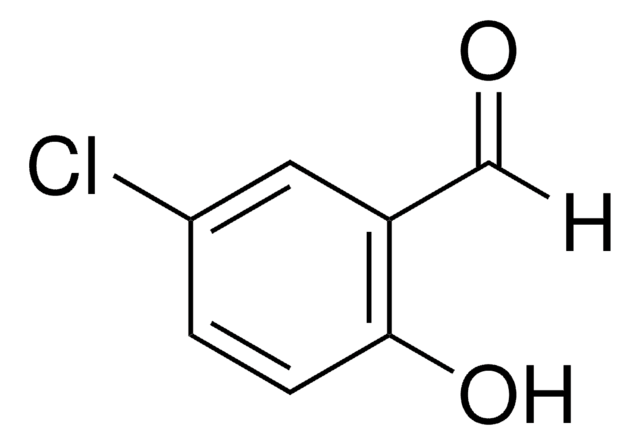
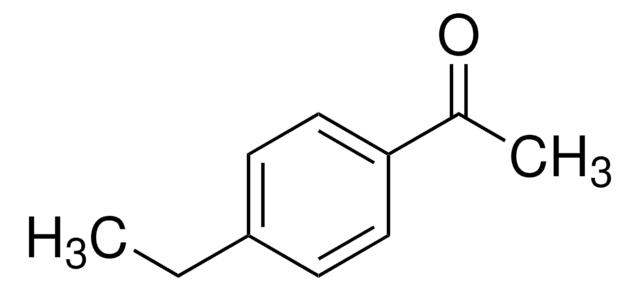
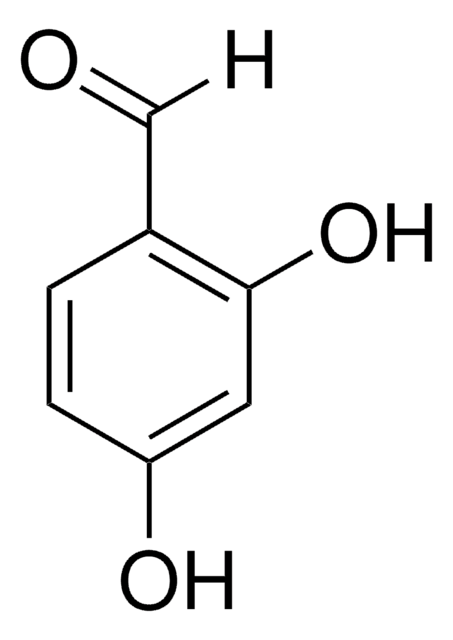
233633
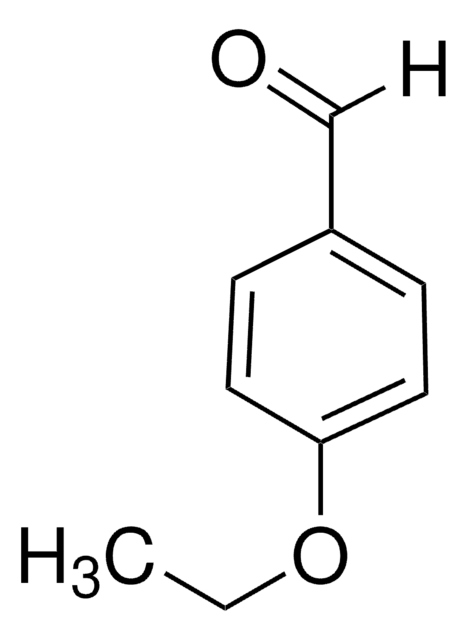
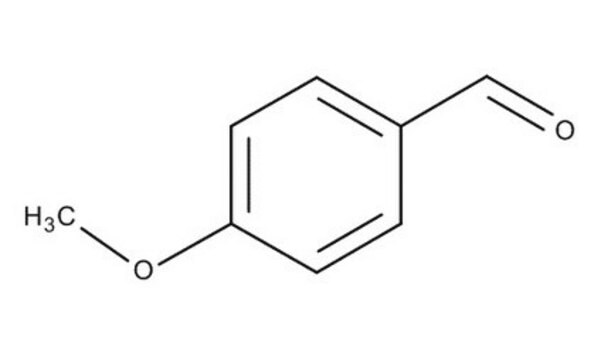
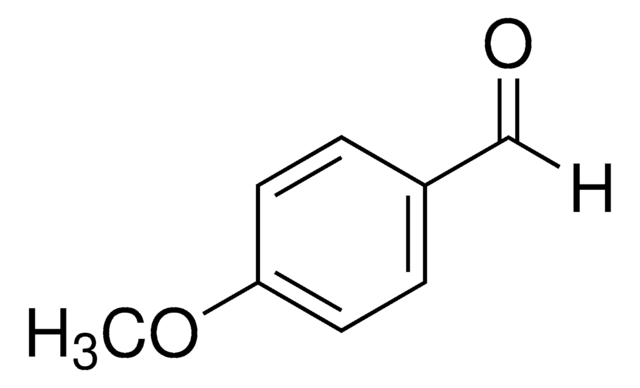
4-Ethylbenzaldehyde
98%
Sinonimo/i:
p-Ethylbenzaldehyde
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C2H5C6H4CHO
Numero CAS:
Peso molecolare:
134.18
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
liquid
Indice di rifrazione
n20/D 1.539 (lit.)
P. ebollizione
221 °C (lit.)
Densità
0.979 g/mL at 25 °C (lit.)
Gruppo funzionale
aldehyde
Stringa SMILE
[H]C(=O)c1ccc(CC)cc1
InChI
1S/C9H10O/c1-2-8-3-5-9(7-10)6-4-8/h3-7H,2H2,1H3
QNGNSVIICDLXHT-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
4-Ethylbenzaldehyde is a by-product of disinfection. Kinetic constants (KI) of 4-ethylbenzaldehyde for inhibition of the diphenolase activity of mushroom tyrosinase has been investigated.
Applicazioni
4-Ethylbenzaldehyde has been used in the synthesis of 4,4′-diaminotriphenylmethanes under microwave irradiation, which is useful for parallel library syntheses.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
197.6 °F - closed cup
Punto d’infiammabilità (°C)
92 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Microwave-assisted synthesis of 4, 4'-diaminotriphenylmethanes.
Guzman-Lucero D, et al.
Tetrahedron Letters, 46(7), 1119-1122 (2005)
Gergely Rácz et al.
Pathology oncology research : POR, 18(3), 579-584 (2011-12-14)
Disinfection of raw water is essential to the production of drinking water. However, by-products of disinfection may exert toxic effects. The potential toxic effects of two of these compounds, 4-ethylbenzaldehyde (EBA) and 2,4-difluoroaniline (DFA) were investigated using the zebrafish (Danio
M Jiménez et al.
Journal of agricultural and food chemistry, 49(8), 4060-4063 (2001-08-22)
A kinetic study of the inhibition of mushroom tyrosinase by 4-substituted benzaldehydes showed that these compounds behave as classical competitive inhibitors, inhibiting the oxidation of L-3,4-dihydroxyphenylalanine (L-DOPA) by mushroom tyrosinase (o-diphenolase activity). The kinetic parameter (K(I)) characterizing this inhibition was
Eduardo Coelho et al.
Food research international (Ottawa, Ont.), 116, 249-257 (2019-02-06)
Cooperage wood is a porous material and beverages exchange compounds with it by penetrating into its pores. This work demonstrates the enrichment of wood with wine during ageing. Three oak varieties were cut into different sized chips and immersed in
Yunzi Feng et al.
Food chemistry, 265, 274-280 (2018-06-10)
Two types of chicken broth, broiler broth (BB) and native chicken broth (NCB), were used to analyse their differences in aroma by gas chromatography-olfactometry/mass spectrometry (GC-O/MS). NCB contained more complex volatiles and exhibited a richer aromatic profile compared with BB.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.