233595
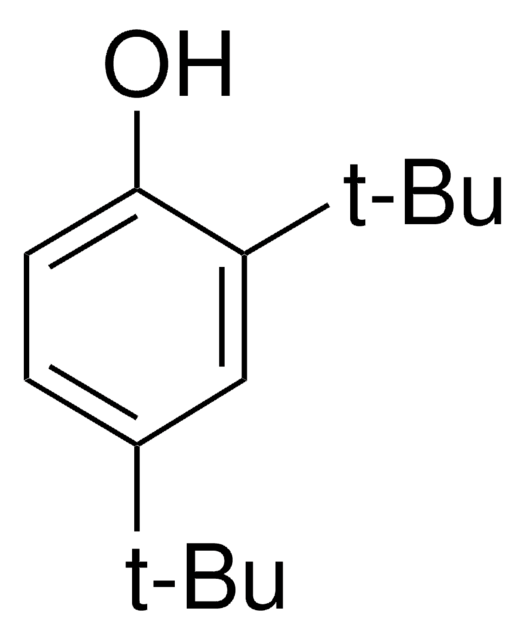
2,6-Diphenylphenol
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(C6H5)2C6H3OH
Numero CAS:
Peso molecolare:
246.30
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Stato
solid
Punto di fusione
101-103 °C (lit.)
Gruppo funzionale
phenyl
Stringa SMILE
Oc1c(cccc1-c2ccccc2)-c3ccccc3
InChI
1S/C18H14O/c19-18-16(14-8-3-1-4-9-14)12-7-13-17(18)15-10-5-2-6-11-15/h1-13,19H
ATGFTMUSEPZNJD-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
2,6-Diphenylphenol reacts with with (n)BuLi, NaH, KH, or Rb or Cs metal in benzene to yield the solvent-free complexes [M(OAr)]x.
Applicazioni
2,6-Diphenylphenol has been used:
- as ligand during the synthesis of reduced coordination (less than 6), unchelated manganese oxygen cluster systems
- in the preparation of derivatives of pyrazine-2,3-dicarbonitrile, precursor required for the synthesis of octaazaphthalocyanine (AzaPc) derivatives
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Acute 1 - Aquatic Chronic 1
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Saad Makhseed et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(16), 4810-4815 (2008-05-01)
The synthesis of octaazaphthalocyanine (AzaPc) derivatives, with bulky phenoxyl substituents placed at eight peripheral positions and containing either H(+), Ni(2+) or Zn(2+) ions in their central cavity, is described. The required precursors, derivatives of pyrazine-2,3-dicarbonitrile, were prepared using a nucleophilic
Charles S Weinert et al.
Inorganic chemistry, 42(19), 6089-6094 (2003-09-16)
Reaction of 2,6-diphenylphenol (HOC(6)H(3)Ph(2)-2,6) with (n)BuLi, NaH, KH, or Rb or Cs metal in benzene gives the solvent-free complexes [M(OAr)]x in excellent yield. The complex [Rb(OC(6)H(3)Ph(2)-2,6)](x)() exhibits a ladderlike structure in the solid state with triply bridging oxygen atoms and
Sandeep K Kondaveeti et al.
Inorganic chemistry, 51(19), 10095-10104 (2012-09-06)
The synthesis of reduced coordination (less than 6), unchelated manganese oxygen cluster systems is described. Addition of phenols to Mn(NR(2))(2) (R = SiMe(3)) results in protolytic amide ligand replacement, and represents the primary entry into the described chemistry. Addition of
Camilo J Viasus et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(68), 17269-17278 (2017-10-17)
The reactivity of carbon dioxide with vanadium(III) aryloxo complexes has been investigated. The formation of either carbon monoxide or incorporation into the ligand system with the ultimate formation of organic ester was observed depending on the overall electron donor ability
Ursula J Williams et al.
Dalton transactions (Cambridge, England : 2003), 43(43), 16197-16206 (2014-08-26)
The trivalent compound K[Ce[N(SiHMe2)2]4] was synthesized and oxidized, providing a convenient route to the reported cerium(IV) compound Ce[N(SiHMe2)2]4. Protonolysis reactions of Ce[N(SiHMe2)2]4 with tert-butanol, substituted benzyl alcohols, and 2,6-diphenylphenol yielded the neutral tetravalent compounds Ce(O(t)Bu)4(py)2, Ce2(OCH2C6R5)8(thf)2 (R = Me, F)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.