229601
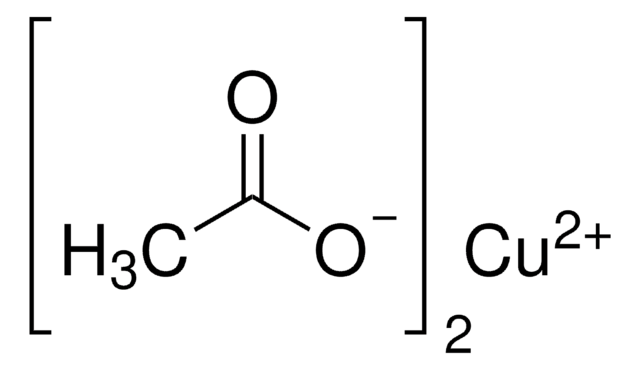
Copper(II) acetate monohydrate
99.99% trace metals basis
Sinonimo/i:
Cupric acetate monohydrate
About This Item
Prodotti consigliati
Densità del vapore
6.8 (vs air)
Livello qualitativo
Saggio
99.99% trace metals basis
Stato
powder or crystals
Impiego in reazioni chimiche
core: copper
Caratteristiche più verdi
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Categoria alternativa più verde
Stringa SMILE
O.CC(=O)O[Cu]OC(C)=O
InChI
1S/2C2H4O2.Cu.H2O/c2*1-2(3)4;;/h2*1H3,(H,3,4);;1H2/q;;+2;/p-2
NWFNSTOSIVLCJA-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- To synthesize CuSbS2 nanoplates and a CuSbS2-Cu3SbS4 nanocomposite via hot injection method. CuSbS2 can be used as an absorber material in solar cells due to its favorable optical properties and direct band gap. The CuSbS2-Cu3SbS4 nanocomposite exhibits promising super capacitive properties, making it suitable for energy storage applications.
- To synthesize copper oxide nanoparticles (CuO NPs) using a green synthesis method involving psidium guajava leaf extract as both a reducing and capping agent. The CuO NPs exhibit excellent photocatalytic activity for degrading industrial dyes, such as Nile Blue (NB) and Reactive Yellow 160 (RY160). These nanoparticles can be used for purifying water resources contaminated with industrial dyes.
- As a copper precursor in synthesizing CuO semiconducting thin films via jet nebulizer spray pyrolysis technique, for P–N diode application. These CuO films find applications in supercapacitors, sensors, solar cells, photocatalysis and electrochromic devices.
Caratteristiche e vantaggi
- It is soluble in water makes a perfect precursor for the synthesis of new materials by sol-gel method
- With high purity of 99.99% (<150 ppm) and low heavy metals, it is ideal for Ir-catalyzed intramolecular C-H amination and the synthesis of carbinol.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8B - Non-combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
does not flash
Punto d’infiammabilità (°C)
does not flash
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
In this article, we will discuss coinage metal deposition processes in order to provide a sense of the most critical precursors, reducing agents, and processes.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.