197637
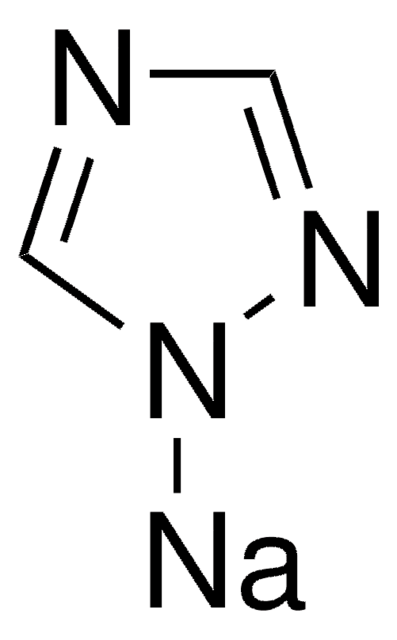
Imidazole sodium derivative
technical grade
Sinonimo/i:
Imidazolylsodium, Sodium imidazolide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C3H3N2Na
Numero CAS:
Peso molecolare:
90.06
Beilstein:
3569312
Numero CE:
Numero MDL:
Codice UNSPSC:
12352005
eCl@ss:
39161001
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Grado
technical grade
Livello qualitativo
Punto di fusione
284 °C (dec.) (lit.)
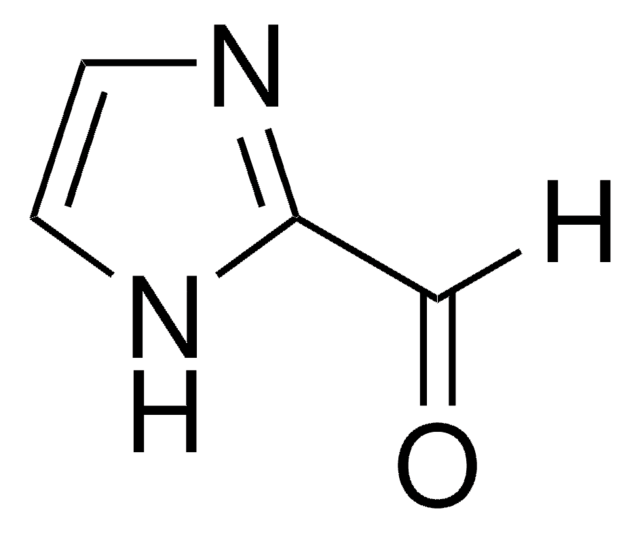
Stringa SMILE
[Na]n1ccnc1
InChI
1S/C3H3N2.Na/c1-2-5-3-4-1;/h1-3H;/q-1;+1
ITAWMPSVROAMOE-UHFFFAOYSA-N
Applicazioni
Imidazole sodium derivative (Imidazolylsodium) was used in the synthesis of arylazidoamorphigenin.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
F G Earley et al.
The Biochemical journal, 224(2), 525-534 (1984-12-01)
A photoaffinity-labelling analogue of the respiratory inhibitor rotenone was synthesized from the naturally occurring rotenoid amorphigenin. The analogue inhibits NADH-ubiquinone oxidoreductase activity at concentrations comparable with those of rotenone. Photolysis of the radiolabelled analogue bound to isolated NADH-ubiquinone oxidoreductase resulted
Richard C Knighton et al.
Chemical communications (Cambridge, England), 49(23), 2293-2295 (2013-02-15)
The dansyl fluorophore ligated to gold nanoparticles via imidazole and amine groups affords conjugates capable of detecting micromolar concentrations of the chemical warfare agent sulfur mustard by a fluorescence switching 'ON' displacement assay.
Yin Gao et al.
Bioorganic & medicinal chemistry, 21(5), 1305-1311 (2013-02-05)
Galactosyltransferases (GalTs) extend the glycan chains of mammalian glycoproteins by adding Gal to terminal GlcNAc residues, and thus build the scaffolds for biologically important glycan structures. We have shown that positively charged bivalent imidazolium salts in which the two imidazolium
Chuanjiang Hu et al.
Inorganic chemistry, 52(6), 3170-3177 (2013-03-09)
The effects of the deprotonation of coordinated imidazole on the vibrational dynamics of five-coordinate high-spin iron(II) porphyrinates have been investigated using nuclear resonance vibrational spectroscopy. Two complexes have been studied in detail with both powder and oriented single-crystal measurements. Changes
Ho Young Lee et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(14), 5416-5421 (2013-03-16)
RNA-binding proteins control the fate and function of the transcriptome in all cells. Here we present technology for isolating RNA-protein partners efficiently and accurately using an engineered clustered regularly interspaced short palindromic repeats (CRISPR) endoribonuclease. An inactive version of the
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.