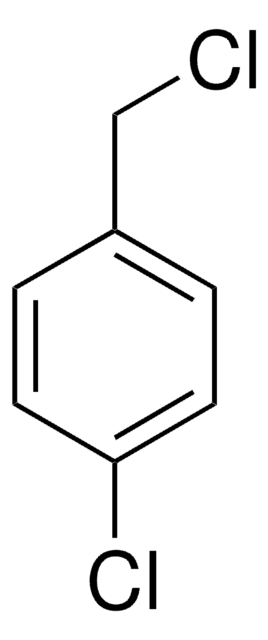
187062
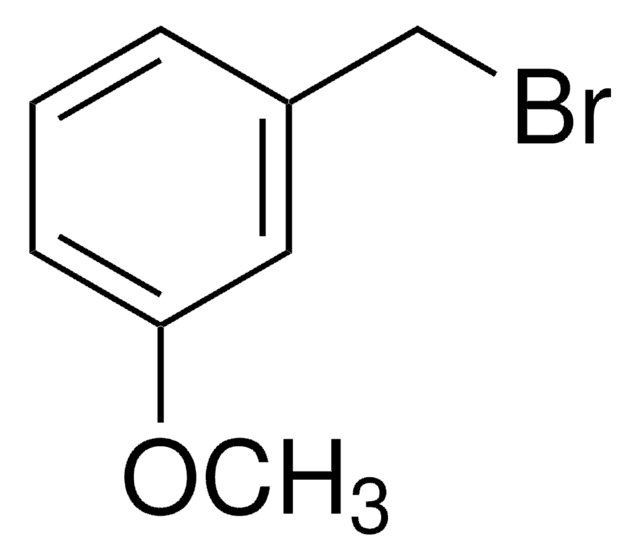
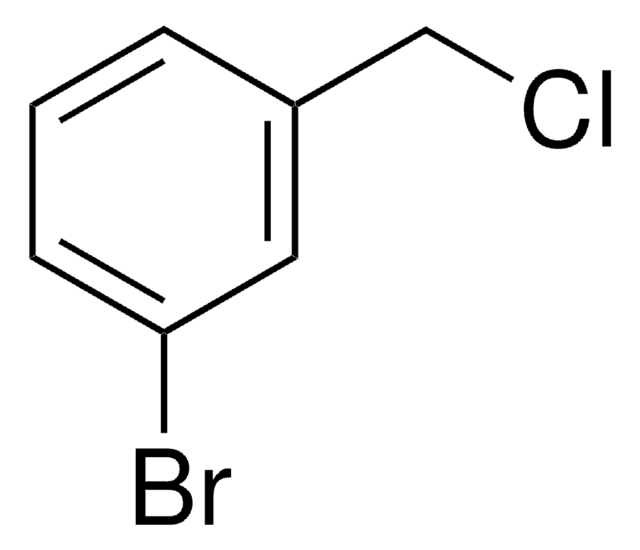
3-Bromobenzyl bromide
99%
Sinonimo/i:
α,3-Dibromotoluene
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
BrC6H4CH2Br
Numero CAS:
Peso molecolare:
249.93
Beilstein:
2078683
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Stato
solid
Punto di fusione
39-41 °C (lit.)
Gruppo funzionale
bromo
Stringa SMILE
BrCc1cccc(Br)c1
InChI
1S/C7H6Br2/c8-5-6-2-1-3-7(9)4-6/h1-4H,5H2
ZPCJPJQUVRIILS-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
3-Bromobenzyl bromide undergoes reduction with diethylzinc in the presence of Pd(PPh3)4 to yield corresponding hydrocarbon.
Applicazioni
3-Bromobenzyl bromide was used in the synthesis of:
- 1,7-di(3-bromobenzyl)cyclen
- substituted 8-arylquinoline, phosphodiesterase 4 (PDE4) inhibitors
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Synthesis of a new family of bi-and polycyclic compounds via Pd-catalyzed amination of 1, 7-di (3-bromobenzyl) cyclen.
Averin AD, et al.
Tetrahedron Letters, 49(24), 3950-3954 (2008)
Reduction of benzylic halides with diethylzinc using tetrakis (triphenylphosphine) palladium as catalyst.
Agrios KA and Srebnik M.
The Journal of Organic Chemistry, 58(24), 6908-6910 (1993)
Dwight Macdonald et al.
Bioorganic & medicinal chemistry letters, 15(23), 5241-5246 (2005-09-20)
The discovery and SAR of a new series of substituted 8-arylquinoline PDE4 inhibitors are herein described. This work has led to the identification of several compounds with excellent in vitro and in vivo profiles, including a good therapeutic window of
William L Scott et al.
Journal of combinatorial chemistry, 11(1), 14-33 (2008-12-25)
Distributed Drug Discovery (D(3)) proposes solving large drug discovery problems by breaking them into smaller units for processing at multiple sites. A key component of the synthetic and computational stages of D(3) is the global rehearsal of prospective reagents and
William L Scott et al.
Journal of combinatorial chemistry, 11(1), 34-43 (2008-12-25)
For the successful implementation of Distributed Drug Discovery (D(3)) (outlined in the accompanying Perspective), students, in the course of their educational laboratories, must be able to reproducibly make new, high quality, molecules with potential for biological activity. This article reports
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.