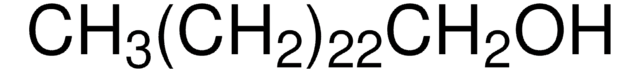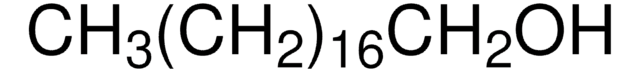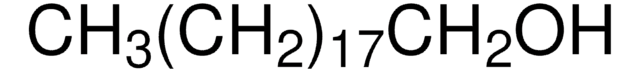169102
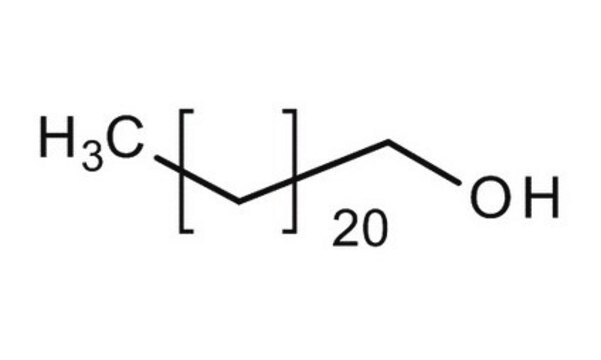
1-Docosanol
98%
Sinonimo/i:
Behenyl alcohol
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3(CH2)21OH
Numero CAS:
Peso molecolare:
326.60
Beilstein:
1770470
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
P. ebollizione
180 °C/0.22 mmHg (lit.)
Punto di fusione
65-72 °C (lit.)
Gruppo funzionale
hydroxyl
Stringa SMILE
CCCCCCCCCCCCCCCCCCCCCCO
InChI
1S/C22H46O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23/h23H,2-22H2,1H3
NOPFSRXAKWQILS-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
1-Docosanol inhibits replication of certain viruses (herpes simplex virus and respiratory syncytial virus) within primary target cells in vitro. It has been isolated from Clematis brevicaudata.
Applicazioni
1-Docosanol was used in the synthesis of series of amphiphilic dendrimers with hydrophilic aliphatic polyether-type dendritic core and hydrophobic docosyl peripheries.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
nwg
Punto d’infiammabilità (°F)
410.0 °F
Punto d’infiammabilità (°C)
210 °C
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Ai-Mei Yang et al.
Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials, 32(10), 1534-1537 (2010-02-02)
To study the chemical constituents from Clematis brevicaudata. The compounds were isolated by column chromatography and their structures were elucidated through spectroscopic analysis (NMR). Eight compounds were isolated and identified as: palmitic acid (1), 1-docosanol (2), pentacosanoic acid-2', 3'-dihydroxypropyl ester
Synthesis and self-assembly of amphiphilic dendrimers based on aliphatic polyether-type dendritic cores.
Cho B-K, et al.
Macromolecules, 37(11), 4227-4234 (2004)
Antiviral activity of 1-docosanol, an inhibitor of lipid-enveloped viruses including herpes simplex.
D H Katz et al.
Proceedings of the National Academy of Sciences of the United States of America, 88(23), 10825-10829 (1991-12-01)
This article reports that 1-docosanol, a 22-carbon-long saturated alcohol, exerts a substantial inhibitory effect on replication of certain viruses (e.g., herpes simplex virus and respiratory syncytial virus) within primary target cells in vitro. To study the basis for its viral
John F Marcelletti
Antiviral research, 56(2), 153-166 (2002-10-09)
Interactions between docosanol (n-docosanol, behenyl alcohol) and nucleoside or pyrophosphate analogs were investigated in vitro. The anti-HSV activity of acyclovir (ACV) was synergistically enhanced by treatment of cells with docosanol as judged by inhibition of progeny virus production and plaque
Clara L Shaw et al.
Journal of chemical ecology, 37(4), 329-339 (2011-03-23)
The uropygial secretions of some bird species contain volatile and semivolatile compounds that are hypothesized to serve as chemical signals. The abundance of secretion components varies with age and season, although these effects have not been investigated in many species.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.