156019
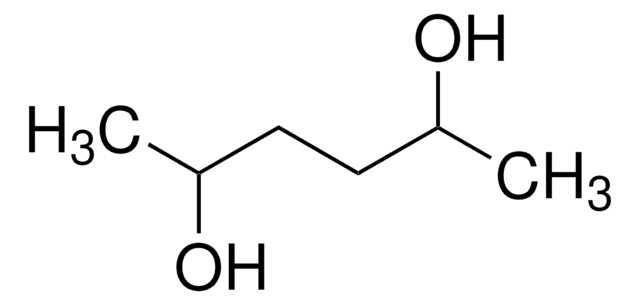
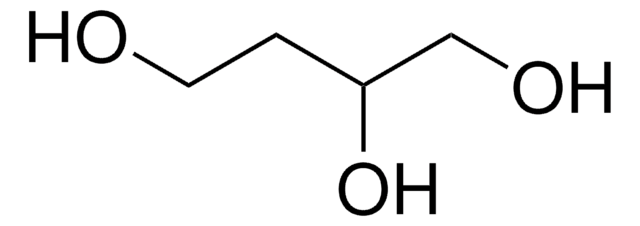
2,4-Pentanediol
98%
Sinonimo/i:
1,3-Dimethylpropane-1,3-diol, 2,4-Amylene glycol, 2,4-Dihydroxypentane
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3CH(OH)CH2CH(OH)CH3
Numero CAS:
Peso molecolare:
104.15
Beilstein:
969186
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
liquid
Indice di rifrazione
n20/D 1.435 (lit.)
P. ebollizione
201-202 °C (lit.)
Densità
0.95 g/mL at 25 °C (lit.)
Gruppo funzionale
hydroxyl
Stringa SMILE
CC(O)CC(C)O
InChI
1S/C5H12O2/c1-4(6)3-5(2)7/h4-7H,3H2,1-2H3
GTCCGKPBSJZVRZ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
2,4-Pentanediol was used in the synthesis of chelated multinuclear complexes.
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
213.8 °F - closed cup
Punto d’infiammabilità (°C)
101.00 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Katsumasa Kamiya et al.
The journal of physical chemistry. A, 116(4), 1168-1175 (2012-01-10)
We report ab initio molecular dynamics calculations based on density functional theory performed on an intramolecular [2 + 2] cycloaddition between ketene and olefin linked with a 2,4-pentanediol (PD) tether. We find that the encounter of the ketene and olefin
P W Tas et al.
Biochimica et biophysica acta, 1026(1), 40-42 (1990-07-09)
Lack of selectivity towards anesthetic stereoisomers is one of the few criteria available for the identification of an anesthetic target site. Until now this criterion has not been tested for anesthetics that directly interact with sensitive membrane proteins which are
Eric J Bierschenk et al.
Inorganic chemistry, 50(23), 12126-12132 (2011-11-08)
When 2,4-pentanediol (2,4-H(2)pd) is deprotonated, the resulting dianion (2,4-pd) serves as a type of "hybrid" ligand, i.e., an alkoxide that possesses structural features of a β-diketonate. 2,4-Pentanediol reacts with Al(O-s-Bu)(3) and Zr(O-i-Pr)(4) to form chelated multinuclear complexes. The aluminum-containing product
Jun-Ichi Matsuo et al.
Organic letters, 12(10), 2294-2297 (2010-04-23)
Ketones and acyl silanes were reduced to the corresponding alcohols by a simple procedure employing anti-1,3-diol and a catalytic amount (5 mol %) of 2,4-dinitrobenzenesulfonic acid in benzene at reflux. Asymmetric induction reached up to >99% ee when a chiral
Belén Martín-Matute et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(23), 6053-6061 (2006-06-27)
Highly efficient synthesis of enantiopure diacetates of 2,4-pentanediol and 2,5-hexanediol starting from commercially available mixtures of the diols (dl/meso approximately 1:1) has been realized by combining a fast ruthenium-catalyzed epimerization with an enzymatic transesterification. The in situ coupling of these
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.