155721
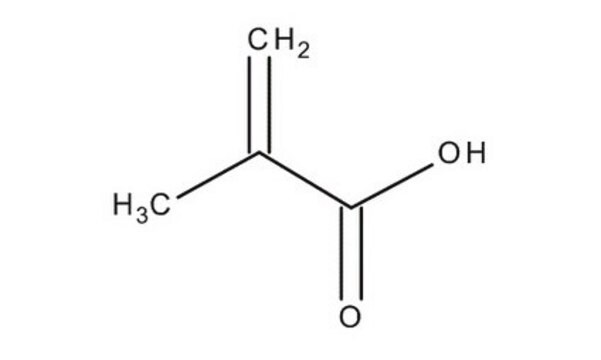
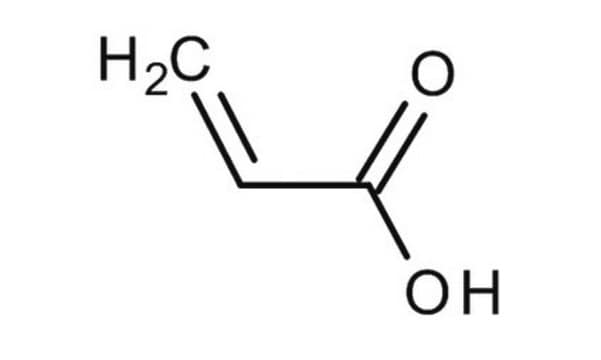
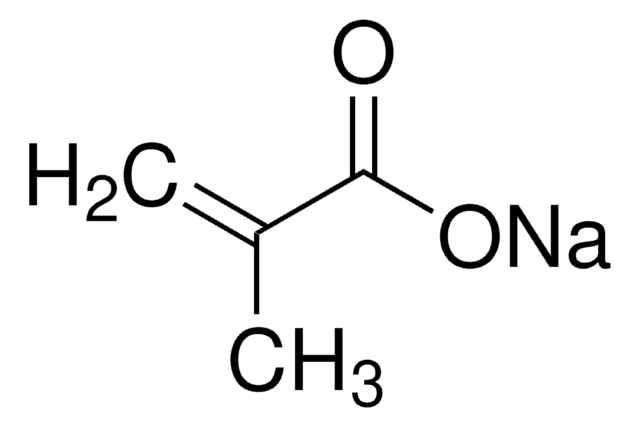
Methacrylic acid
contains 250 ppm MEHQ as inhibitor, 99%
Sinonimo/i:
2-Methacrylic acid, 2-Methylpropenoic acid
About This Item
Prodotti consigliati
Densità del vapore
>3 (vs air)
Livello qualitativo
Tensione di vapore
1 mmHg ( 20 °C)
Saggio
99%
Forma fisica
liquid
Temp. autoaccensione
752 °F
contiene
250 ppm MEHQ as inhibitor
Indice di rifrazione
n20/D 1.431 (lit.)
pH
2.0-2.2 (20 °C, 100 g/L)
P. eboll.
163 °C (lit.)
Punto di fusione
12-16 °C (lit.)
Densità
1.015 g/mL at 25 °C (lit.)
Stringa SMILE
C=C(C)C(O)=O
InChI
1S/C4H6O2/c1-3(2)4(5)6/h1H2,2H3,(H,5,6)
CERQOIWHTDAKMF-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1A - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
152.6 °F - closed cup
Punto d’infiammabilità (°C)
67 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
(RAFT) Polymerization
Composites
Articoli
RAFT (Reversible Addition Fragmentation chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
The manufacture of monomers for use in ophthalmic applications is driven by the need for higher purity, improved reliability of manufacturing supply, but ultimately by the need for the increased comfort, convenience, and safety of contact lens wearers. Daily wear contact lenses have the potential to fill this need for many customers; however, their widespread use is constrained by higher costs compared to weekly- or monthly-based lenses. New approaches that improve cost structure and result in higher quality raw materials are needed to help make contact lenses more affordable and accelerate growth of the contact lens market.
By altering the physicochemical properties, smart or intelligent drug delivery systems can be designed to deliver therapeutic molecules on-demand. Learn more about the application of stimuli-responsive materials in drug delivery.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.