146188
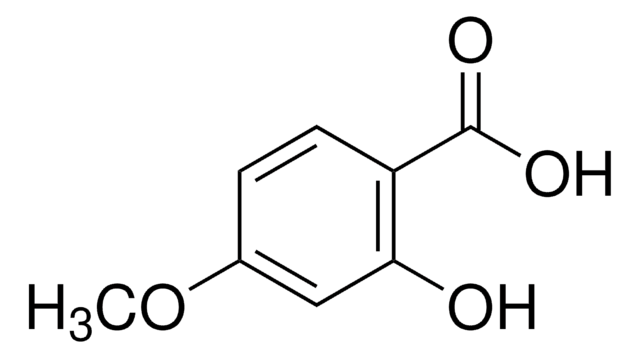
2-Hydroxy-5-methoxybenzoic acid
98%
Sinonimo/i:
5-Methoxysalicylic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3OC6H3(OH)CO2H
Numero CAS:
Peso molecolare:
168.15
Beilstein:
2209647
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Forma fisica
powder
Punto di fusione
141-143 °C (lit.)
Stringa SMILE
COc1ccc(O)c(c1)C(O)=O
InChI
1S/C8H8O4/c1-12-5-2-3-7(9)6(4-5)8(10)11/h2-4,9H,1H3,(H,10,11)
IZZIWIAOVZOBLF-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
2-Hydroxy-5-methoxybenzoic acid is matrix additive which enhances the electrical conductivity of the matrix crystal during matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS).
Applicazioni
2-Hydroxy-5-methoxybenzoic acid was used to evaluate MALDI matrix/solvent combinations for intact spore mass spectrometry of Fusarium species.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Maximilianos Kotsias et al.
PloS one, 14(1), e0210759-e0210759 (2019-01-18)
Protein O-glycosylation has shown to be critical for a wide range of biological processes, resulting in an increased interest in studying the alterations in O-glycosylation patterns of biological samples as disease biomarkers as well as for patient stratification and personalized
Beatriz Gonçalves et al.
Journal of experimental botany, 68(21-22), 5801-5811 (2017-12-01)
The CUP-SHAPED COTYLEDON (CUC) transcription factors control plant boundary formation, thus allowing the emergence of novel growth axes. While the developmental roles of the CUC genes in different organs and across species are well characterized, upstream and downstream events that
Kathrin Stavenhagen et al.
Molecular & cellular proteomics : MCP, 17(6), 1225-1238 (2017-12-14)
Human C1-inhibitor (C1-Inh) is a serine protease inhibitor and the major regulator of the contact activation pathway as well as the classical and lectin complement pathways. It is known to be a highly glycosylated plasma glycoprotein. However, both the structural
Stephanie Holst et al.
Scientific reports, 7(1), 16623-16623 (2017-12-02)
To characterise pancreatic cancer cells from different sources which are used as model systems to study the metastatic behaviour in pancreatic ductal adenocarcinoma (PDAC), we compared the N-glycan imprint of four PDAC cells which were previously shown to differ in
Jasmin Kemptner et al.
Rapid communications in mass spectrometry : RCM, 23(6), 877-884 (2009-02-19)
Unambiguous identification of mycotoxin-producing fungal species as Fusarium is of great relevance to agriculture and the food-producing industry as well as in medicine. Protein profiles of intact fungal spores, such as Penicillium, Aspergillus and Trichoderma, derived from matrix-assisted laser desorption/ionization
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.