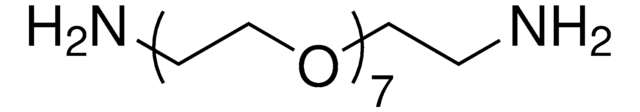14502
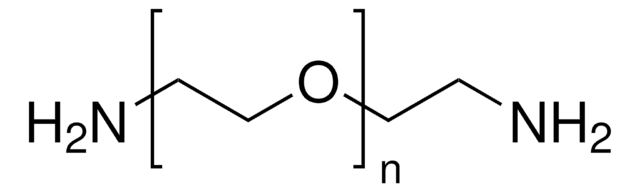
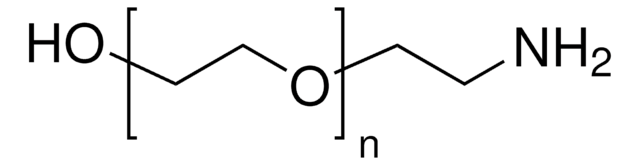
Poly(ethylene glycol) bis(amine)
Mw 3,000, carboxyl reactive, amine
Sinonimo/i:
Polyethylene glycol, O,O′-Bis(2-aminoethyl)polyethylene glycol, Diaminopolyethylene glycol, PEG-diamine, Polyoxyethylene bis(amine)
About This Item
Prodotti consigliati
Nome del prodotto
Poly(ethylene glycol) bis(amine), Mw 3,000
PM
Mw 3,000
Livello qualitativo
Impiego in reazioni chimiche
reagent type: cross-linking reagent
reactivity: carboxyl reactive
Estremità Ω
amine
Estremità α
amine
Architettura del polimero
shape: linear
functionality: homobifunctional
InChI
1S/C6H16N2O2/c7-1-3-9-5-6-10-4-2-8/h1-8H2
IWBOPFCKHIJFMS-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Progress in biotechnology fields such as tissue engineering and drug delivery is accompanied by an increasing demand for diverse functional biomaterials. One class of biomaterials that has been the subject of intense research interest is hydrogels, because they closely mimic the natural environment of cells, both chemically and physically and therefore can be used as support to grow cells. This article specifically discusses poly(ethylene glycol) (PEG) hydrogels, which are good for biological applications because they do not generally elicit an immune response. PEGs offer a readily available, easy to modify polymer for widespread use in hydrogel fabrication, including 2D and 3D scaffold for tissue culture. The degradable linkages also enable a variety of applications for release of therapeutic agents.
Devising biomaterial scaffolds that are capable of recapitulating critical aspects of the complex extracellular nature of living tissues in a threedimensional (3D) fashion is a challenging requirement in the field of tissue engineering and regenerative medicine.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.