11627
Diethyl azodicarboxylate solution
purum, ~40% in toluene (H-NMR)
Sinonimo/i:
1,2-Ethoxycarbonyl diazene solution, DEAD, Diethoxycarbonyldiazene solution, Diethyl azodiformate solution, NSC 3474, NSC 679015, Unifoam AZ-AE 200
About This Item
Prodotti consigliati
Grado
purum
Livello qualitativo
Forma fisica
solid
Concentrazione
~40% in toluene (H-NMR)
Indice di rifrazione
n20/D 1.47
Temperatura di conservazione
2-8°C
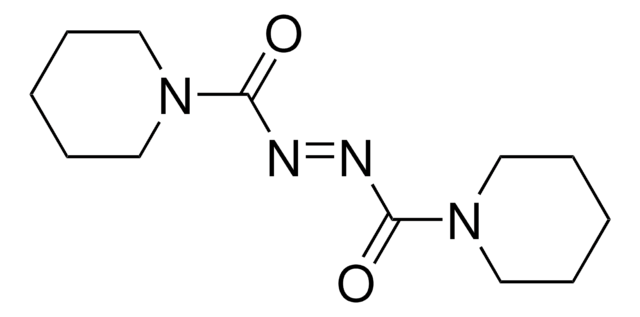
Stringa SMILE
CCOC(=O)\N=N/C(=O)OCC
InChI
1S/C6H10N2O4/c1-3-11-5(9)7-8-6(10)12-4-2/h3-4H2,1-2H3/b8-7+
FAMRKDQNMBBFBR-BQYQJAHWSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
DEAD can also be used as a reagent in the:
- Synthesis of esters, ethers, amines, and thioethers of alcohols.
- Oxidation of alcohols to carbonyl derivatives using ZnBr2 as a catalyst via dehydrogenation reaction.
- Conversion of alcohol to an azide key intermediate in the total synthesis of immunostimulant α-galactosylceramides.
- Synthesis of aza-β-lactams from aryl(alkyl)ketenes.
- Synthesis of 1H-1,2,4-triazole-1,4(5H)-dicarboxylate derivatives.
- Diels-Alder type reactions.
- Immunostimulants α-Galactosylceramides
- Cellotriose and cellotetraose analogues as transition state mimics for mechanistic studies of cellulases
- Bisubstrate inhibitors with molecular recognition at the active site of catechol-O-methyltransferase
- Derivatives of F200 and S383 with cannabinoid CB1 receptor binding activities
- Aza-β-lactams via NHC-catalyzed [2 + 2] cycloaddition with ketenes
Reagent for:
- Annulation of N-protected imines
- α-thiocyanation of enolizable ketones with ammonium thiocyanate
- Diels-Alder reactions
Altre note
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 3 - Repr. 2 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
Organi bersaglio
Central nervous system, Respiratory system
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
105.8 °F - closed cup
Punto d’infiammabilità (°C)
41 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.