914134
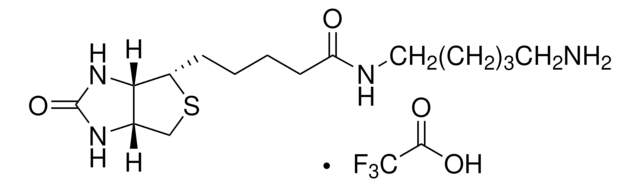
5-(Biotinamido)pentylamine TFA Salt
≥95%
Synonym(s):
N-(5-Aminopentyl)-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pent, Biotin cadaverine TFA, Biotin-DAPe TFA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H28N4O2S · xC2HF3O2
Molecular Weight:
328.47 (free base basis)
MDL number:
UNSPSC Code:
12352116
NACRES:
NA.22
form:
powder
Assay:
≥95%
Recommended Products
Quality Level
Assay
≥95%
form
powder
mp
116-121 °C
storage temp.
2-8°C
SMILES string
FC(F)(F)C(=O)O.S1[C@H]([C@H]2NC(=O)N[C@H]2C1)CCCCC(=O)NCCCCCN
Application
5-(Biotinamido)pentylamine TFA Salt is a versatile biotinylated linker that can be incorporated into chemical tools via its terminal amino group. Labeling materials or proteins with biotin provides a means to enrich and capture targets from biological systems.
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Other Notes
Site-selective conversion of azido groups at carbonyl α-positions into oxime groups leading triazide to a triple click conjugation scaffold
Convergent synthesis of trifunctional molecules by three sequential azido-type-selective cycloadditions
Locked by Design: A Conformationally Constrained Transglutaminase Tag Enables Efficient Site-Specific Conjugation
Synthesis of Novel Phosphonic-Type Activity-Based Probes for Neutrophil Serine Proteases and Their Application in Spleen Lysates of Different Organisms
Convergent synthesis of trifunctional molecules by three sequential azido-type-selective cycloadditions
Locked by Design: A Conformationally Constrained Transglutaminase Tag Enables Efficient Site-Specific Conjugation
Synthesis of Novel Phosphonic-Type Activity-Based Probes for Neutrophil Serine Proteases and Their Application in Spleen Lysates of Different Organisms
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sung Kook Chun et al.
ACS chemical biology, 9(3), 703-710 (2014-01-07)
Circadian rhythms, biological oscillations with a period of about 24 h, are maintained by a genetically determined innate time-keeping system called the molecular circadian clockwork. Despite the physiological and clinical importance of the circadian clock, the development of small molecule
Marianne van Wyk et al.
Chemical communications (Cambridge, England), (4), 398-400 (2007-01-16)
Coenzyme A analogues are synthesized in a one-pot preparation by biotransformation of pantothenate thioesters through the simultaneous use of three CoA biosynthetic enzymes, followed by aminolysis.
Taiki Yokoi et al.
Chemical communications (Cambridge, England), 55(13), 1891-1894 (2018-12-21)
This paper reports the selective conversion of alkyl azido groups at the carbonyl α-position into oximes through β-elimination of dinitrogen, followed by transoximation. With this method and diazo conversion, a triazido molecule was transformed into a triple click conjugation scaffold
Collette S Guy et al.
Organic & biomolecular chemistry, 17(43), 9524-9528 (2019-10-30)
Dimeric benzoboroxoles that are covalently linked by a short scaffold enhance selective anti-tubercular activity. These multimeric benzoboroxole compounds are capable of engaging the specific extracellular Mycobacterium tuberculosis glycans, do not lead to the evolution of resistance and bypass the need
Aoi Teraoka et al.
Chemical communications (Cambridge, England), 50(6), 664-666 (2013-11-28)
A facile and useful method for preparing caged DNAs was developed. The method includes a caging reaction of a linear dsDNA having a minimal sequence of protein expression with Bio-Bhc-diazo and affinity separation of the caged DNA. Effective suppression and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service