805408
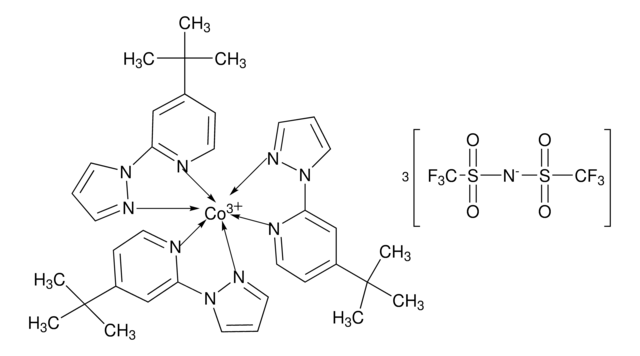
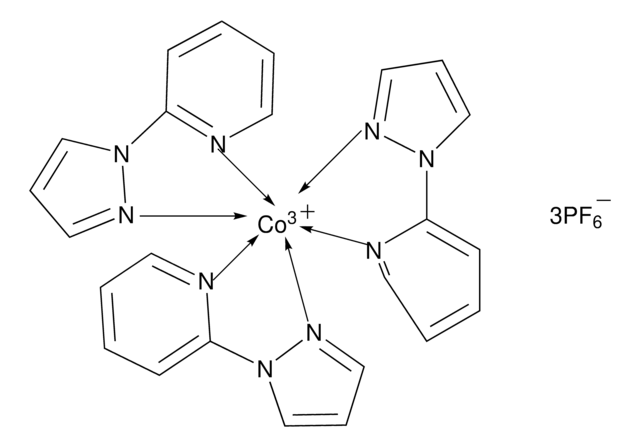
FK 209 Co(III) PF6 salt
Synonym(s):
Greatcell Solar®, tris(2-(1H-pyrazol-1-yl)-4-tert-butylpyridine)cobalt(III) tri[hexafluorophosphate]
About This Item
Recommended Products
Assay
98%
Quality Level
form
powder
SMILES string
CC(C)(C)C1=CC(N2N=CC=C2)=NC=C1.CC(C)(C)C3=CC=NC(N4C=CC=N4)=C3.CC(C)(C)C5=CC=NC(N6C=CC=N6)=C5.[Co+3]
InChI
1S/3C12H15N3.Co/c3*1-12(2,3)10-5-7-13-11(9-10)15-8-4-6-14-15;/h3*4-9H,1-3H3;/q;;;+3
InChI key
GECNGXUHOILCGK-UHFFFAOYSA-N
Application
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Next generation solar cells have the potential to achieve conversion efficiencies beyond the Shockley-Queisser (S-Q) limit while also significantly lowering production costs.
Dr. Perini and Professor Correa-Baena discuss the latest research and effort to obtain higher performance and stability of perovskite materials.
For several decades, the need for an environmentally sustainable and commercially viable source of energy has driven extensive research aimed at achieving high efficiency power generation systems that can be manufactured at low cost.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service