推荐产品
等級
pharmaceutical primary standard
API 家族
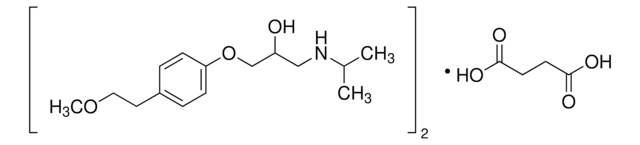
metoprolol
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
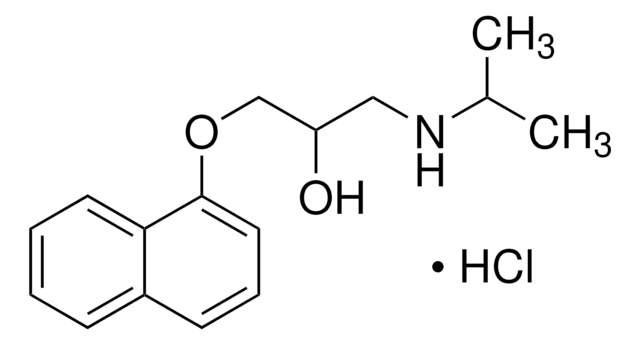
O[C@H]([C@@H](O)C(O)=O)C(O)=O.COCCc1ccc(OCC(O)CNC(C)C)cc1.COCCc2ccc(OCC(O)CNC(C)C)cc2
InChI
1S/2C15H25NO3.C4H6O6/c2*1-12(2)16-10-14(17)11-19-15-6-4-13(5-7-15)8-9-18-3;5-1(3(7)8)2(6)4(9)10/h2*4-7,12,14,16-17H,8-11H2,1-3H3;1-2,5-6H,(H,7,8)(H,9,10)/t;;1-,2-/m..1/s1
InChI 密鑰
YGULWPYYGQCFMP-CEAXSRTFSA-N
基因資訊
human ... ADRB1(153)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Metoprolol tartrate USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Metoprolol Tartrate and Hydrochlorothiazide Tablets
- Metoprolol Tartrate Compounded Oral Solution
- Metoprolol Tartrate Compounded Oral Suspension
- Metoprolol Tartrate Injection
- Metoprolol Tartrate Tablets
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Aquatic Chronic 2 - Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
C Narendra et al.
AAPS PharmSciTech, 7(2), E34-E34 (2006-06-27)
The purpose of the present study was to develop an optimized gastric floating drug delivery system (GFDDS) containing metoprolol tartrate (MT) as a model drug by the optimization technique. A 2(3) factorial design was employed in formulating the GFDDS with
Design and evaluation of mucoadhesive buccal drug delivery systems containing metoprolol tartrate.
Ramana, M. V., C. Nagda, and M. Himaja.
Indian Journal of Pharmaceutical Sciences, 69.4, 515-515 (2007)
Wenhuan Li et al.
Medicine, 93(28), e329-e329 (2014-12-20)
The sensitivity and specificity of 5 different image sets of dual-energy computed tomography (DECT) for the detection of first-pass myocardial perfusion defects have not systematically been compared using positron emission tomography (PET) as a reference standard. Forty-nine consecutive patients, with
I S Hamadeh et al.
Clinical pharmacology and therapeutics, 96(2), 175-181 (2014-03-19)
Metoprolol is a selective β-1 adrenergic receptor blocker that undergoes extensive metabolism by the polymorphic enzyme cytochrome P450 2D6 (CYP2D6). Our objective was to investigate the influence of CYP2D6 polymorphisms on the efficacy and tolerability of metoprolol tartrate. Two hundred
Rahil H Kassamali et al.
AJR. American journal of roentgenology, 203(4), 759-762 (2014-09-24)
The purpose of this study was to assess the safety of heart rate optimization by use of β-adrenergic blockade solely by the i.v. route before coronary CT angiography. The records of 679 patients undergoing CT coronary angiography after receiving i.v.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![(±)-1-(乙胺基)-3-[4-(2-甲氧基乙基)苯氧基] -2-丙醇 United States Pharmacopeia (USP) Reference Standard](/deepweb/assets/sigmaaldrich/product/structures/111/137/a4e919d4-6638-4cb5-84fd-6e5f9da972da/640/a4e919d4-6638-4cb5-84fd-6e5f9da972da.png)