推荐产品
品質等級
化驗
≥98% (HPLC)
形狀
powder
光學活性
[α]/D -980 to -1015 (C=1, MeOH)
顏色
yellow
溶解度
DMSO: ≥25 mg/mL
儲存溫度
2-8°C
SMILES 字串
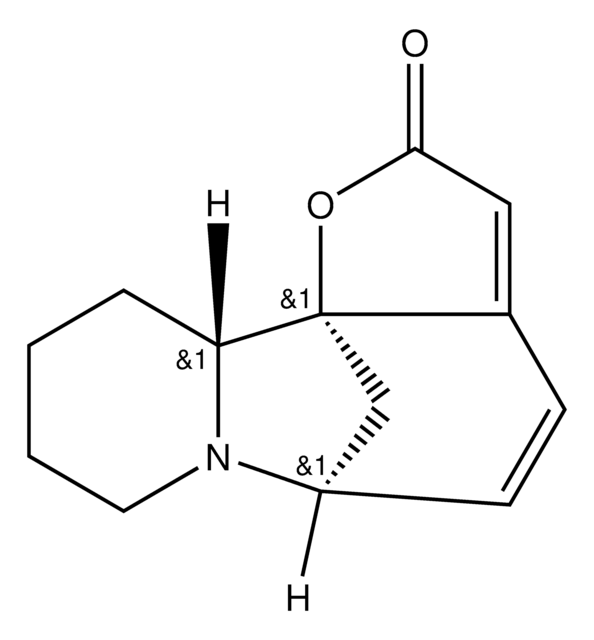
O=C1O[C@@]23C[C@@H](C=CC2=C1)N4CCCC[C@H]34
InChI
1S/C13H15NO2/c15-12-7-9-4-5-10-8-13(9,16-12)11-3-1-2-6-14(10)11/h4-5,7,10-11H,1-3,6,8H2/t10-,11-,13+/m1/s1
InChI 密鑰
SWZMSZQQJRKFBP-WZRBSPASSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Securinine was used as a standard in the synthesis of the members of Securinega alkaloid family.
生化/生理作用
Securinine is an alkaloid widely in traditional folk medicine. Long known as a GABAA antagonist, securinine was recently found to up-regulate p53 protein and to modulate the related family member p73 protein in a p53-dependent fashion, inducing p73 in the HCT116 p53(-) cells and down-regulating it in the p53(+) cells. Induction of proapoptotic protein p73 only in p53 cells could be used to target cancer cells preferentially. Securinine has been found to induce p53-independent, p73-dependent apoptosis in RKO colon cancer cells.
特點和優勢
This compound is featured on the GABAA Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
H Pu et al.
Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials, 24(4), 278-280 (2003-02-18)
Pharmacological activities of virosecurinine (Vse) and securinine (Sec) were studied. The results showed that acute toxicity of Vse was 1/13.6 that of Sec, and Vse had no convulsive effects on rats or frogs, while Sec had. The results also showed
David González-Gálvez et al.
The Journal of organic chemistry, 74(16), 6199-6211 (2009-07-25)
The most representative securinega alkaloids have been synthesized through a new strategy involving the palladium-catalyzed enantioselective allylation of a cyclic imide, a vinylogous Mannich reaction, and a ring-closing metathesis process, as the key steps. The diastereoselectivity of the vinylogous Mannich
Kirk Lubick et al.
Journal of leukocyte biology, 82(5), 1062-1069 (2007-08-19)
Innate immune cell stimulation represents a complementary approach to vaccines and antimicrobial drugs to counter infectious disease. We have used assays of macrophage activation and in vitro and in vivo phase II Coxiella burnetii infection models to compare and contrast
Michael Holmes et al.
Experimental parasitology, 127(2), 370-375 (2010-09-14)
Securinine, an alkaloid originally isolated from Securinega suffruticosa, exhibits a wide range of biological activities, including anti-malarial activity. Along with securinine, 10 pyrrolidine derivatives, generated via the retrosynthesis of (-)-securinine, were selected and tested for their inhibitory activity against Toxoplasma
Jung-Hsuan Chen et al.
Organic letters, 14(17), 4531-4533 (2012-08-24)
Total syntheses of (±)-securinine and (±)-allosecurinine that employ a tandem rhodium carbenoid-initiated Claisen/α-ketol rearrangement sequence as a key step are described.
商品
We offer many products related to GABAA receptors for your research needs.
We offer many products related to GABAA receptors for your research needs.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门