推荐产品
形狀
solid
品質等級
顏色
white
溶解度
DMSO: 2 mg/mL
2-hydroxypropyl-β-cyclodextrin: insoluble
H2O: insoluble
ethanol: insoluble
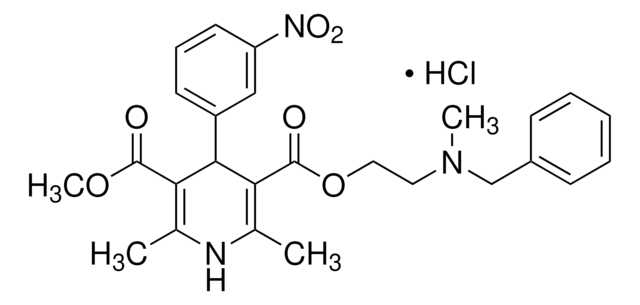
SMILES 字串
Clc1ccc2nc(NC(=O)Cc3ccccc3)n4nc(nc4c2c1)-c5ccco5
InChI
1S/C21H14ClN5O2/c22-14-8-9-16-15(12-14)20-25-19(17-7-4-10-29-17)26-27(20)21(23-16)24-18(28)11-13-5-2-1-3-6-13/h1-10,12H,11H2,(H,23,24,28)
InChI 密鑰
TWWFAXQOKNBUCR-UHFFFAOYSA-N
基因資訊
human ... ADORA3(140)
rat ... Adora1(29290) , Adora2a(25369) , Adora3(25370)
生化/生理作用
MRS1220 is a putative A3 adenosine receptor antagonist. MRS 1220 was found to be competitive in saturation binding studies using the agonist radioligand 125I AB-MECA at cloned human brain A3 receptors expressed in HEK-293 cells. Antagonism was demonstrated in functional assays consisting of agonist-induced inhibition of adenylate cyclase and the stimulation of binding of 35S guanosine 5′-O-(3-thiotriphosphate (35S GTP-gamma-S) to the associated G-proteins. MRS 1220 and MRS 1191, with KB values of 1.7 and 92 nM, respectively, proved to be highly selective for human A3 receptor vs human A1 receptor-mediated effects on adenylate cyclase. In addition, MRS 1220 reversed the effect of A3 agonist-elicited inhibition of tumor necrosis factor-alpha formation in the human macrophage U-937 cell line, with an IC50 value of 0.3 μM.
特點和優勢
This compound is featured on the Adenosine Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Naunyn-Schmiedeberg'S Archives of Pharmacology, 364, 225-234 (2000)
Pnina Fishman et al.
Arthritis research & therapy, 8(1), R33-R33 (2006-03-02)
The anti-inflammatory effect of adenosine was previously found to be mediated via activation of the A3 adenosine receptor (A3AR). The aim of the present study was to decipher the molecular mechanism involved with the inhibitory effect of IB-MECA, an A3AR
Yaara Gorzalczany et al.
Translational oncology, 12(12), 1549-1556 (2019-09-08)
We have recently shown that mast cells (MCs), which constitute an important part of the tumor microenvironment (TME), can be directly activated by cancer cells under conditions that recapitulate cell to cell contact. However, MCs are often detected in the
K A Jacobson et al.
Neuropharmacology, 36(9), 1157-1165 (1997-11-19)
The effects of putative A3 adenosine receptor antagonists of three diverse chemical classes (the flavonoid MRS 1067, the 6-phenyl-1,4-dihydropyridines MRS 1097 and MRS 1191, and the triazoloquinazoline MRS 1220) were characterized in receptor binding and functional assays. MRS1067, MRS 1191
Y C Kim et al.
Journal of medicinal chemistry, 39(21), 4142-4148 (1996-10-11)
The adenosine antagonist 9-chloro-2-(2-furanyl)[1,2,4]triazolo[1,5-c]quinazolin-5-amine (CGS15943) binds to human A3 receptors with high affinity (Ki = 14 nM), while it lacks affinity at rat A3 receptors. Acylated derivatives of the 5-amino group and other modifications were prepared in an effort to
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门