所有图片(2)
About This Item
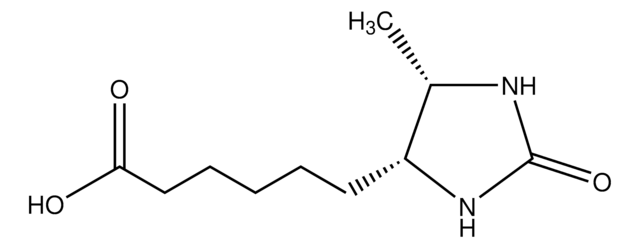
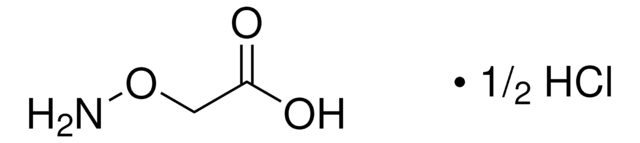
经验公式(希尔记法):
C10H17N3O2S
CAS号:
分子量:
243.33
Beilstein:
18952
MDL號碼:
分類程式碼代碼:
12352203
PubChem物質ID:
NACRES:
NA.46
推荐产品
生物源
synthetic (organic)
品質等級
化驗
≥98% (TLC)
形狀
powder
溶解度
1 M HCl: 50 mg/mL, clear, colorless
儲存溫度
2-8°C
SMILES 字串
[H][C@]12CS[C@@H](CCCCC(O)=O)[C@@]1([H])NC(=N)N2
InChI
1S/C10H17N3O2S/c11-10-12-6-5-16-7(9(6)13-10)3-1-2-4-8(14)15/h6-7,9H,1-5H2,(H,14,15)(H3,11,12,13)/t6-,7-,9-/m0/s1
InChI 密鑰
WWVANQJRLPIHNS-ZKWXMUAHSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2-亚氨基生物素是生物素的环状胍基类似物,在高 pH (> 9) 下对亲和素具有高亲和力,在低 pH (< 6) 下具有低亲和力。亚氨基生物素化的蛋白质选择性地包含在 pH 9-11 的亲和素柱内,可在 pH 4 洗脱或加入生物素洗脱。该技术可用于研究细胞表面蛋白,通过标记和选择性地从完整的人红细胞表面回收高碘酸盐敏感的成分。2-亚氨基生物素通过其胍基可逆性抑制 NOS(一氧化氮合酶)。它抑制 NO 的生物合成,了解这类重要酶的结合位点相互作用可能很重要。NOS 氧化 L-精氨酸的胍基氮生成一氧化氮和 L-瓜氨酸。
應用
已将 2-亚氨基生物素用作竞争性抑制剂,与链霉亲和素的生物结合位点结合,以恢复荧光信号。
免責聲明
除非我们的产品目录或产品附带的其他公司文档另有说明,否则我们的产品仅供研究使用,不得用于任何其他目的,包括但不限于未经授权的商业用途、体外诊断用途、离体或体内治疗用途或任何类型的消费或应用于人类或动物。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Hiroyuki Inoue et al.
Langmuir : the ACS journal of surfaces and colloids, 21(18), 8354-8359 (2005-08-24)
Layered thin films composed of avidin and 2-iminobiotin-labeled poly(ethyleneimine) (ib-PEI) were prepared by a layer-by-layer deposition of avidin and ib-PEI on a solid surface, and the disintegration induced by changing environmental pH and adding biotin in the solution was studied.
F Garret-Flaudy et al.
Biotechnology and bioengineering, 71(3), 223-234 (2001-04-06)
Affinity precipitation, especially secondary effect affinity precipitation, has repeatedly been suggested as a valuable technique for the biotechnical downstream process. The present lack of applications is related to the scarcity of predictable affinity macroligands and to the fact that rather
Amesh B Patel et al.
Journal of the American Chemical Society, 126(5), 1318-1319 (2004-02-05)
The strength of a multimolecular system depends on the number of interactions that hold it together. Using dynamic force spectroscopy, we show how the kinetic stability of a system decreases as the number of molecular bonds is increased, as predicted
Cora H A Nijboer et al.
Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 27(2), 282-292 (2006-06-01)
We have shown earlier that 2-iminobiotin (2-IB) reduces hypoxia-ischemia (HI)-induced brain damage in neonatal rats, and presumed that inhibition of nitric oxide synthases (NOS) was the underlying mechanism. We now investigated the effect of 2-IB treatment in P7 rat pups
Changlian Zhu et al.
Journal of neurochemistry, 90(2), 462-471 (2004-07-02)
Excessive nitric oxide (NO) production after cerebral hypoxia-ischaemia (HI) may induce cellular injury in various ways, including reaction with superoxide to form the highly reactive peroxynitrite. We characterized the spatial and temporal formation of peroxynitrite through immunohistochemical detection of nitrosylated
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门