推荐产品
品質等級
化驗
≥90%
儲存溫度
−20°C
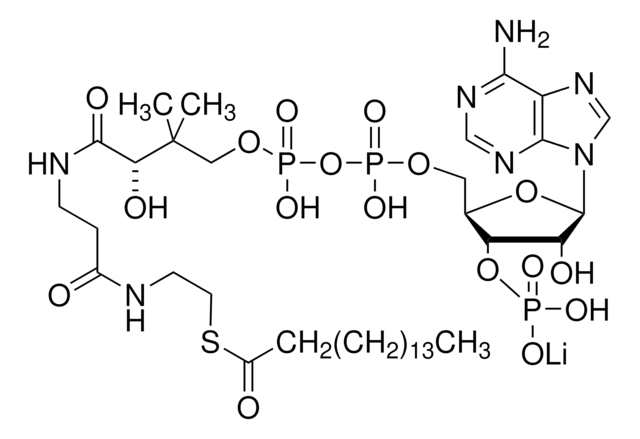
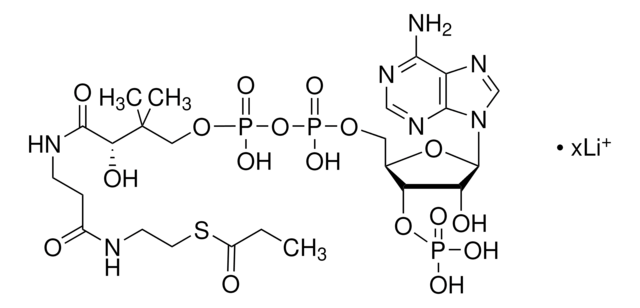
SMILES 字串
[Li].CC(C)(COP(O)(=O)OP(O)(=O)OCC1OC(C(O)C1OP(O)(O)=O)n2cnc3c(N)ncnc23)C(O)C(=O)NCCC(=O)NCCSC(=O)CCCC(O)=O
InChI
1S/C26H42N7O19P3S/c1-26(2,21(39)24(40)29-7-6-15(34)28-8-9-56-17(37)5-3-4-16(35)36)11-49-55(46,47)52-54(44,45)48-10-14-20(51-53(41,42)43)19(38)25(50-14)33-13-32-18-22(27)30-12-31-23(18)33/h12-14,19-21,25,38-39H,3-11H2,1-2H3,(H,28,34)(H,29,40)(H,35,36)(H,44,45)(H,46,47)(H2,27,30,31)(H2,41,42,43)/t14-,19-,20-,21?,25-/m1/s1
InChI 密鑰
SYKWLIJQEHRDNH-KRPIADGTSA-N
一般說明
戊二酰辅酶A(戊二酰CoA)是线粒体氧化赖氨酸、羟赖氨酸和色氨酸的中间体。
應用
戊二酰辅酶A锂盐已被用于:
- 通过定量蛋白质组学
- 对β-羟基β-甲基戊二酰-CoA(HMG-CoA)和戊二酰-CoA的酰基化进行比较性研究,作为FapR-NLuc蛋白
- 体外生物传感器活性实验的测定缓冲液的组成部分,以测试其对a549裂解物中丙酮酸激酶活性抑制的效应
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Jörg Schaarschmidt et al.
FEBS letters, 585(9), 1317-1321 (2011-04-12)
Glutaryl-coenzyme A (CoA) dehydrogenases (GDHs) are acyl-CoA dehydrogenases, which usually dehydrogenate and decarboxylate the substrate to crotonyl-CoA. In some anaerobic bacteria, non-decarboxylating GDHs exist that release glutaconyl-CoA (2,3-dehydroglutaryl-CoA) without decarboxylation. The differing mechanisms of decarboxylating and non-decarboxylating GDHs were investigated
Structural basis for promoting and preventing decarboxylation in glutaryl-coenzyme a dehydrogenases.
Simon Wischgoll et al.
Biochemistry, 49(25), 5350-5357 (2010-05-22)
Glutaryl-coenzyme A dehydrogenases (GDHs) involved in amino acid degradation were thought to catalyze both the dehydrogenation and decarboxylation of glutaryl-coenzyme A to crotonyl-coenzyme A and CO(2). Recently, a structurally related but nondecarboxylating, glutaconyl-coenzyme A-forming GDH was characterized in the obligately
A Class of Reactive Acyl-CoA Species Reveals the Non-enzymatic Origins of Protein Acylation
Wagner GR, et al.
Cell Metabolism, 25(4), 823-837 (2017)
Kevin A Strauss et al.
Brain : a journal of neurology, 133(Pt 1), 76-92 (2009-12-25)
In glutaric aciduria type 1, glutaryl-coenzyme A and its derivatives are produced from intracerebral lysine and entrapped at high concentrations within the brain, where they interfere with energy metabolism. Biochemical toxicity is thought to trigger stroke-like striatal degeneration in susceptible
Discovering targets of non-enzymatic acylation by thioester reactivity profiling
Kulkarni RA, et al.
Cell Chemical Biology, 24(2), 231-242 (2017)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门