所有图片(1)
About This Item
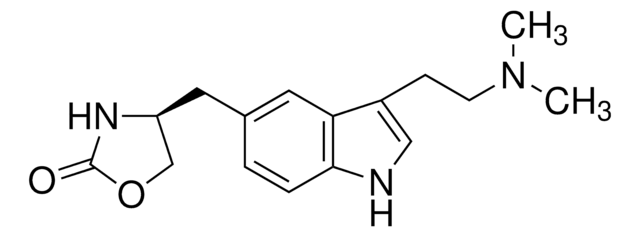
经验公式(希尔记法):
C19H27N3O4S
CAS号:
分子量:
393.50
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推荐产品
化驗
≥98% (HPLC)
形狀
solid
顏色
white to light pink
溶解度
DMSO: >20 mg/mL
起源
GlaxoSmithKline
儲存溫度
2-8°C
SMILES 字串
Cn1cc(C(=O)OCC2CCN(CCNS(C)(=O)=O)CC2)c3ccccc13
InChI
1S/C19H27N3O4S/c1-21-13-17(16-5-3-4-6-18(16)21)19(23)26-14-15-7-10-22(11-8-15)12-9-20-27(2,24)25/h3-6,13,15,20H,7-12,14H2,1-2H3
InChI 密鑰
MOZPSIXKYJUTKI-UHFFFAOYSA-N
基因資訊
human ... HTR4(3360)
rat ... Htr3a(79246) , Htr4(25324)
應用
GR 113808已被用作5-羟色胺受体4(5-HT4R)阻断剂,以研究其对大鼠心室心肌细胞中钙(Ca2+)瞬变的影响。它也已被用作5-HT4R拮抗剂,以研究其对胚胎后斑马鱼从头肠神经发生的影响。
生化/生理作用
GR 113808是5-HT4血清素受体拮抗剂。
特點和優勢
该化合物由 GlaxoSmithKline开发。想浏览其他制药公司开发的化合物和已批准药物/候选药物目录, 请单击此处。
法律資訊
根据GlaxoSmithKline的协议出售用于研究目的
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
A N James et al.
Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society, 17(1), 76-82 (2005-01-27)
Selective serotonin reuptake inhibitors (SSRIs) are increasingly used to treat a variety of disorders but have gastrointestinal side-effects. To determine the effects of the SSRI, fluoxetine, on gastric smooth muscle contractility. Fundic, antral, and pyloric circular muscle contractility of guinea
Lucie Rivail et al.
British journal of pharmacology, 143(3), 361-370 (2004-09-08)
A body of evidences suggests that a hydrophobic pocket of the human 5-HT(4) receptor contributes to the high affinity of some bulky 5-HT(4) ligands. A thorough study of this pocket was performed using mutagenesis and molecular modeling. Ligand binding or
Guillaume Lucas et al.
Biological psychiatry, 57(8), 918-925 (2005-04-12)
We recently identified a facilitory control exerted by serotonin4 (5-HT4) receptors on the in vivo firing activity of dorsal raphe nucleus (DRN) serotonergic (5-HT) neurons. However, these findings were based on acute administrations of 5-HT4 receptor agonists and antagonists, which
J Kamei et al.
Neuroscience, 174, 224-233 (2010-11-18)
Respiratory depression is the most well-known and dangerous side-effect of opioid analgesics. Clinical investigations have revealed that this opioid-induced respiratory depression is less severe in patients with chronic pain, but the mechanisms that underlie this phenomenon are unknown. Therefore, the
Tadayoshi Mikami et al.
Journal of pharmacological sciences, 107(3), 251-259 (2008-07-01)
In the present study, binding affinities of 5-hydroxytryptamine-4 (5-HT(4)) ligands for the human 5-HT(4d) receptor were determined using the agonist [(3)H]5-HT and the selective 5-HT(4) antagonist [(3)H]GR113,808. We also compared the affinity differences between [(3)H]5-HT binding (K(H)) and [(3)H]GR113,808 binding
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门