推荐产品
品質等級
化驗
≥98%
技術
HPLC: suitable
gas chromatography (GC): suitable
mp
185-188 °C (lit.)
應用
forensics and toxicology
pharmaceutical (small molecule)
veterinary
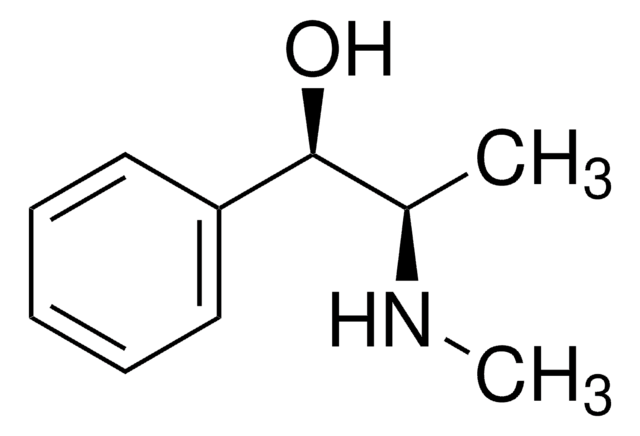
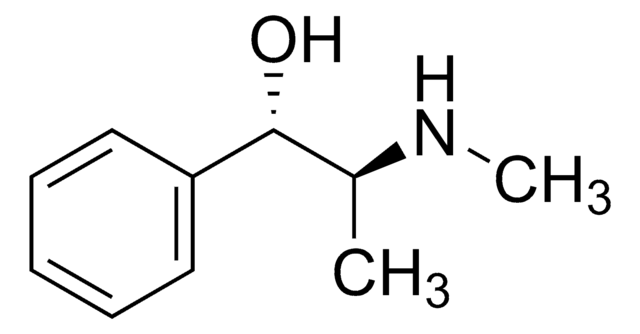
SMILES 字串
Cl.CN[C@@H](C)[C@@H](O)c1ccccc1
InChI
1S/C10H15NO.ClH/c1-8(11-2)10(12)9-6-4-3-5-7-9;/h3-8,10-12H,1-2H3;1H/t8-,10+;/m0./s1
InChI 密鑰
BALXUFOVQVENIU-KXNXZCPBSA-N
正在寻找类似产品? 访问 产品对比指南
生化/生理作用
非选择性肾上腺素激动剂;减充血剂
應用
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
Minjie Jiang et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 974, 126-130 (2014-12-03)
A rapid, sensitive and selective high-performance liquid chromatography-tandem mass spectrometric method (HPLC-MS) has been developed and validated for the simultaneous determination of 14-thienyl methylene matrine (TMM) and matrine (MT) in rat plasma in the present study. The analytes were separated
Minjie Jiang et al.
PloS one, 10(2), e0116010-e0116010 (2015-02-26)
A rapid, sensitive and selective high-performance liquid chromatography-tandem mass spectrometric method (HPLC-MS) was developed and validated to determine the 14-(3-methylbenzyl)matrine (3MBM) and 14-(4-methylbenzyl)matrine (4MBM) levels in rat plasma in the present study. The analytes were separated using a C18 column
Nigel Greene et al.
Chemical research in toxicology, 23(7), 1215-1222 (2010-06-18)
Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals can make a significant contribution to the identification of potential
Zhichao Liu et al.
PLoS computational biology, 7(12), e1002310-e1002310 (2011-12-24)
Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI types (hepatotoxic side effects) seen in the clinic can be translated
Sean Ekins et al.
Drug metabolism and disposition: the biological fate of chemicals, 38(12), 2302-2308 (2010-09-17)
Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predictive in vivo, in vitro, and in silico models to identify compounds
Chromatograms
application for HPLCapplication for HPLCapplication for HPLC我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门