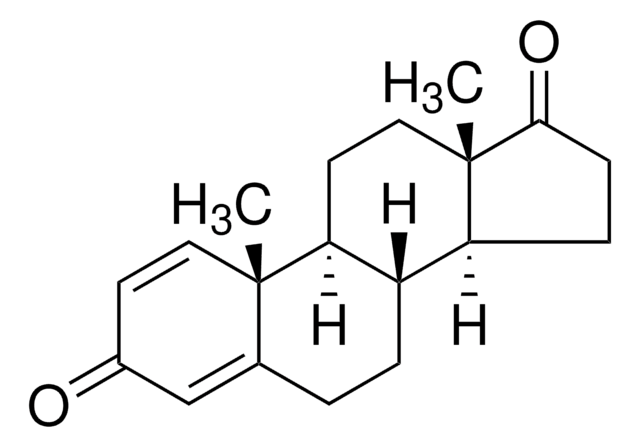
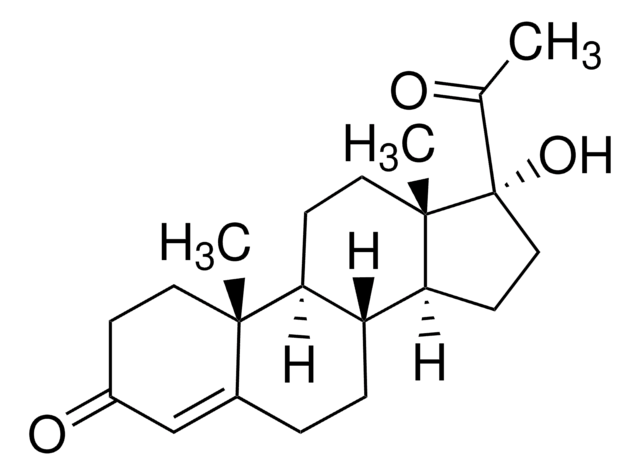
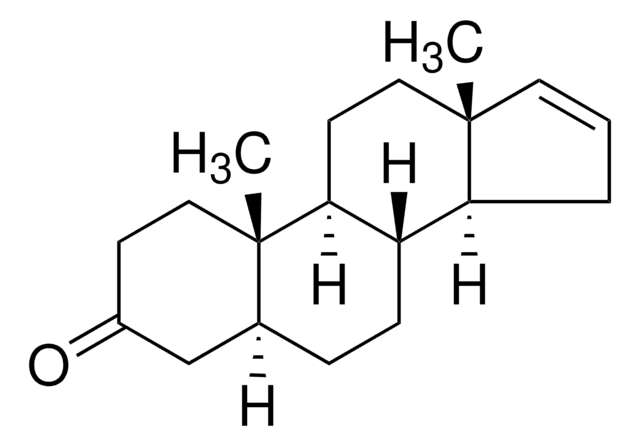
推荐产品
生化/生理作用
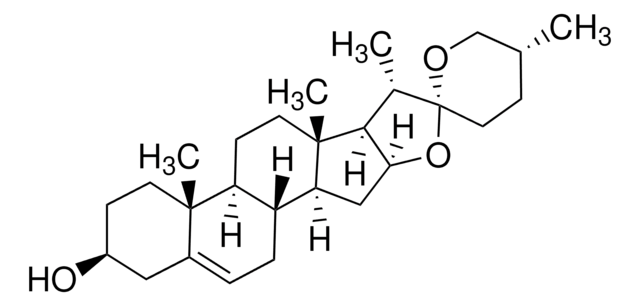
16-Dehydropregnenolone acetate (DPA) is synthesized from steroids sapogenin, diosgenin and solasodine. 16-Dehydropregnenolone acetate (DPA) is a crucial intermediate for the synthesis of steroid hormones-based drugs. It is an antagonist for farnesoid X receptor (FXR) and modulates cholesterol metabolism. It is considered as a potential antihyperlipidemic agent. Chemically synthesized steroid derivatives from DPA have cytotoxic features and could serve as potential anticancer agents.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
The synthesis of 16-dehydropregnenolone acetate (DPA) from potato glycoalkaloids
Vronena PJE, et al.
ARKIVOC (Gainesville, FL, United States), 2(1), 24-50 (2004)
A V Kamernitzky et al.
Journal of steroid biochemistry, 16(1), 61-67 (1982-01-01)
A new class of modified progesterones with an additional ring in the 16 alpha , 17 alpha-position (pregna-D'-pentaranes) are described. Compounds containing 4- and 6-membered D'-ring (D'4- and D'6-pentaranes) were synthesized by the cycloaddition of acetylene or 1,3-butadiene, respectively, to
Víctor Pérez-Ornelas et al.
Steroids, 70(3), 217-224 (2005-03-15)
The enzyme 5alpha-reductase is responsible for the conversion of testosterone (T) to its more potent androgen dihydrotestosterone (DHT). This steroid had been implicated in androgen-dependent diseases such as: benign prostatic hyperplasia, prostate cancer, acne and androgenic alopecia. The inhibition of
16-Dehydropregnenolone lowers serum cholesterol by up-regulation of CYP7A1 in hyperlipidemic male hamsters
Ramakrishna R, et al.
The Journal of Steroid Biochemistry and Molecular Biology, 168(1), 110-117 (2017)
Facile green synthesis of 16-dehydropregnenolone acetate (16-DPA) from diosgenin
Baruah D, et al.
Synthetic Communications, 46(1), 79-84 (2016)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门