推荐产品
化驗
≥97%
形狀
solid
光學活性
[α]26/D +93.6°, c = 6.12 in chloroform(lit.)
顏色
white to off-white
溶解度
methanol: 28 mg/mL(lit.)
DMSO: 3 mg/mL(lit.)
chloroform: 50 mg/mL
ethanol: 6.6 mg/mL(lit.)
dilute aqueous acid and base: insoluble(lit.)
儲存溫度
−20°C
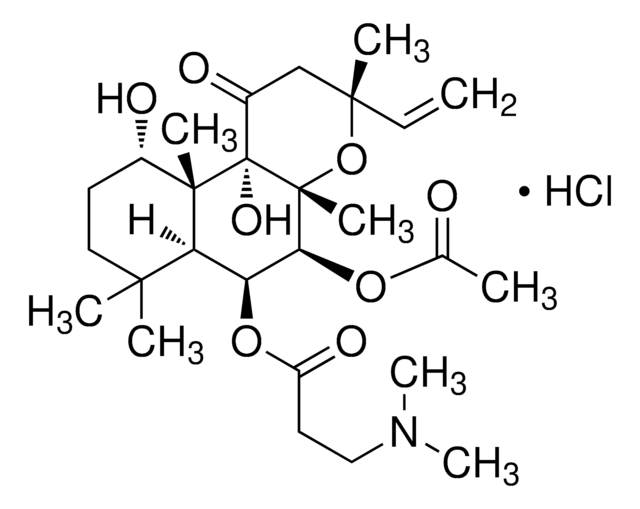
SMILES 字串
[H][C@@]12[C@H](O)[C@H](OC(C)=O)[C@@]3(C)O[C@](C)(CC(=O)C3[C@@]1(C)CCCC2(C)C)C=C
InChI
1S/C22H34O5/c1-8-20(5)12-14(24)16-21(6)11-9-10-19(3,4)17(21)15(25)18(26-13(2)23)22(16,7)27-20/h8,15-18,25H,1,9-12H2,2-7H3/t15-,16?,17-,18-,20-,21+,22-/m0/s1
InChI 密鑰
ZKZMDXUDDJYAIB-OJPJTMFRSA-N
應用
Useful as a negative control for forskolin.
生化/生理作用
Biologically inactive forskolin analog. Does not stimulate adenylyl cyclase.
特點和優勢
This compound is a featured product for Cyclic Nucleotide research. Click here to discover more featured Cyclic Nucleotide products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Adenylyl cyclases page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Hisahiro Hagiwara et al.
The Journal of organic chemistry, 71(12), 4619-4624 (2006-06-06)
Forskolin (1), a highly oxygenated labdane diterpenoid and an activator of adenylate cyclase, has been synthesized in 12 steps and 12% overall yield from ptychantin A (4), which has been isolated from liverwort Ptychanthus striatus in good yield. The 1alpha-hydroxy
Y P Vedernikov et al.
American journal of obstetrics and gynecology, 182(3), 620-624 (2000-03-30)
We sought to study the contribution of potassium channels in the effect of forskolin and 1,9-dideoxyforskolin on uterine contractility in the pregnant rat. Rings taken from the middle portions of uterine horns from rats at 16 days of gestation were
K Schmidt et al.
Journal of cardiovascular pharmacology, 13(3), 353-360 (1989-03-01)
The effects of forskolin and seven derivatives on cardiac functions were investigated by using the Langendorff technique and the results compared with the respective potencies obtained from adenylate cyclase and binding studies. In the isolated heart, forskolin increased all parameters
D Gramaglia et al.
Cell death and differentiation, 11(3), 342-353 (2004-01-10)
Human T-lymphoma Jurkat cells treated with several intrinsic death stimuli readily undergo a stepwise apoptotic program. Treatment with 1,9-dideoxyforskolin (ddFSK), an inactive analogue of the adenylate cyclase activator forskolin, induces necrotic cell death and switches to necrosis the response to
P Wangemann et al.
The Journal of membrane biology, 170(1), 67-77 (1999-07-10)
Receptors were identified pharmacologically in functional studies where K+ secretion was monitored as transepithelial current (Isc). Further, receptors were identified as transcripts by cloning and sequencing of reverse-transcriptase polymerase chain reaction (RT-PCR) products. Isc under control conditions was 796 +/-
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门