所有图片(1)
About This Item
经验公式(希尔记法):
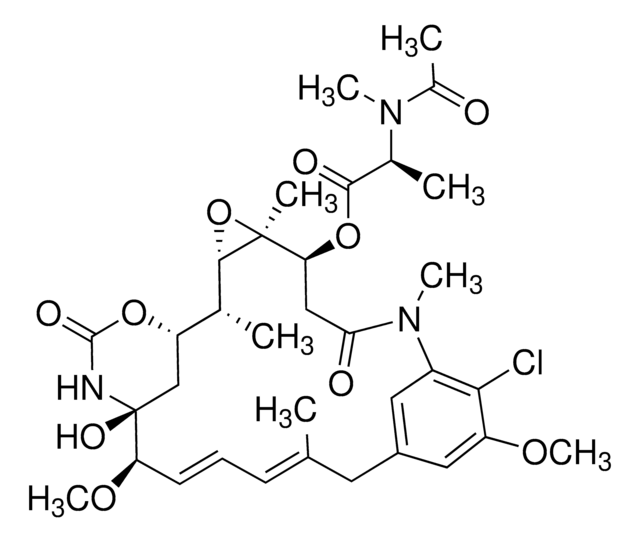
C32H43ClN2O9
CAS号:
分子量:
635.14
MDL號碼:
分類程式碼代碼:
12352202
PubChem物質ID:
NACRES:
NA.32
推荐产品
品質等級
化驗
≥90% (HPLC)
形狀
powder
儲存溫度
2-8°C
SMILES 字串
CO[C@@H]1\C=C\C=C(C)\Cc2cc(OC)c(Cl)c(c2)N(C)C(=O)C[C@H](OC(=O)C(C)C)[C@]3(C)O[C@@H]3[C@H](C)[C@@H]4C[C@@]1(O)NC(=O)O4
InChI
1S/C32H43ClN2O9/c1-17(2)29(37)43-25-15-26(36)35(6)21-13-20(14-22(40-7)27(21)33)12-18(3)10-9-11-24(41-8)32(39)16-23(42-30(38)34-32)19(4)28-31(25,5)44-28/h9-11,13-14,17,19,23-25,28,39H,12,15-16H2,1-8H3,(H,34,38)/b11-9+,18-10+/t19-,23+,24-,25+,28-,31+,32+/m1/s1
InChI 密鑰
OPQNCARIZFLNLF-KUDLRRJMSA-N
生化/生理作用
Fungal metabolite with antineoplastic, antimitotic activity. Binds to tubulin and inhibits vinblastine-induced spiral formation.
訊號詞
Warning
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Carl E Snipes et al.
Journal of natural products, 70(10), 1578-1581 (2007-09-26)
The ansacarbamitocins are a new family of maytansinoids that are unusually substituted with a glucose subunit and two carbamate functional groups and exhibit modest activity against some agricultural fungal disease organisms. Ansacarbamitocins A-F ( 1- 6) all consist of the
Daniel Ng et al.
Journal of industrial microbiology & biotechnology, 36(11), 1345-1351 (2009-07-18)
Constitutive overexpression of regulators in the ansamitocin biosynthetic cluster of Actinosynnema pretiosum was investigated as a strategy to increase the production of ansamitocin-P3 (AP-3), a clinically promising chemotherapeutic agent. Putative transcriptional regulators asm2, asm29, and asm34 as well as the
Highly active ansamitocin derivatives: mutasynthesis using an AHBA-blocked mutant.
Florian Taft et al.
Chembiochem : a European journal of chemical biology, 9(7), 1057-1060 (2008-04-03)
Juan Ma et al.
Archives of pharmacal research, 30(6), 670-673 (2007-08-08)
By using preparative TLC as the critical isolation procedure, two compounds, including one new amide N-glycosides of ansamitocin (2), were isolated from Actinosynnema pretiosum. The compounds were elucidated as N-demethyl-N-beta-D-glucopyranosyl ansamitocin P-2, named ansamitocinoside P-2 (1), and N-demethyl-N-beta-D-glucopyranosyl ansamitocin P-1
Peiji Zhao et al.
Chemistry & biology, 15(8), 863-874 (2008-08-30)
Ansamitocins are potent antitumor maytansinoids produced by Actinosynnema pretiosum. Their biosynthesis involves the initial assembly of a macrolactam polyketide, followed by a series of postpolyketide synthase (PKS) modifications. Three ansamitocin glycosides were isolated from A. pretiosum and fully characterized structurally
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门