推荐产品
品質等級
化驗
≥90% (HPLC)
形狀
solid
雜質
≤12% water
溶解度
H2O: 50 mg/mL, clear, colorless
儲存溫度
−20°C
SMILES 字串
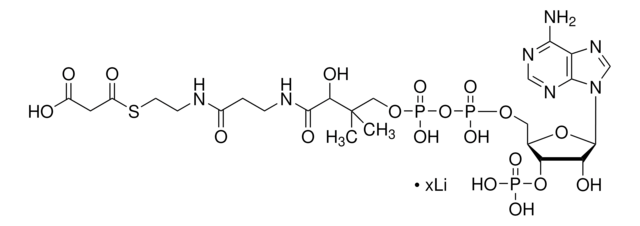
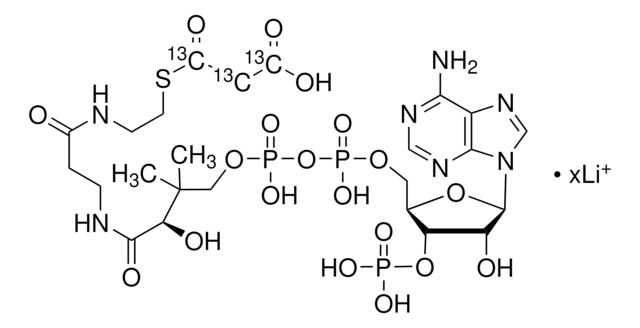
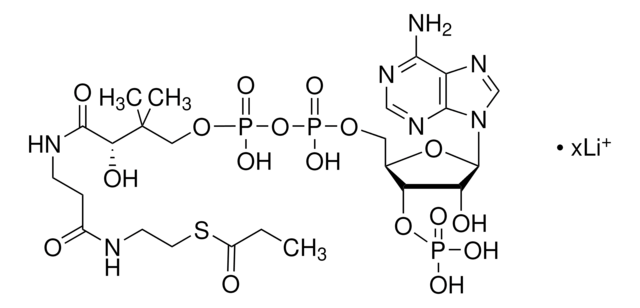
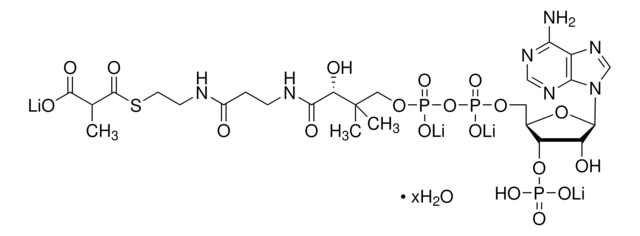
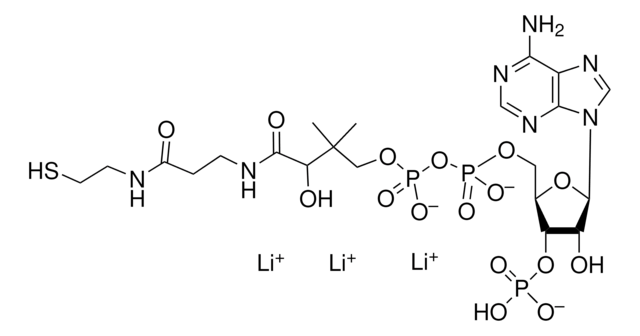
[Li+].[Li+].[Li+].[Li+].CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n2cnc3c(N)ncnc23)[C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC(O)=O
InChI
1S/C24H38N7O19P3S.4Li/c1-24(2,19(37)22(38)27-4-3-13(32)26-5-6-54-15(35)7-14(33)34)9-47-53(44,45)50-52(42,43)46-8-12-18(49-51(39,40)41)17(36)23(48-12)31-11-30-16-20(25)28-10-29-21(16)31;;;;/h10-12,17-19,23,36-37H,3-9H2,1-2H3,(H,26,32)(H,27,38)(H,33,34)(H,42,43)(H,44,45)(H2,25,28,29)(H2,39,40,41);;;;/q;4*+1/p-4/t12-,17-,18-,19+,23-;;;;/m1..../s1
InChI 密鑰
DQLXXYMNINXQSV-QTRRCMKVSA-J
生化/生理作用
丙二酰辅酶A是一种辅酶A衍生物,可用于脂肪酸和聚酮合成,以及胯线粒体膜转移酮戊二酸。丙二酰辅酶A是由乙酰辅酶A羧化酶介导的乙酰辅酶A的羧化反应形成的。
其他說明
丙二酰胺辅酶a在克隆胰岛β细胞葡萄糖刺激的胰岛素分泌中的作用
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Lars Robbel et al.
Biochemistry, 50(27), 6073-6080 (2011-06-10)
Biosynthesis of the hydroxamate-type siderophore erythrochelin requires the generation of δ-N-acetyl-δ-N-hydroxy-L-ornithine (L-haOrn), which is incorporated into the tetrapeptide at positions 1 and 4. Bioinformatic analysis revealed the FAD-dependent monooxygenase EtcB and the bifunctional malonyl-CoA decarboxylase/acetyltransferase Mcd to be putatively involved
Dea Hwan Kim et al.
Plant physiology and biochemistry : PPB, 47(11-12), 991-997 (2009-09-08)
In this study, a flavonoid malonyltransferase (OsMaT-2) was cloned from Oryza sativa, and the recombinant protein OsMaT-2 was purified via affinity chromatography. OsMaT-2 utilized a variety of flavonoid glucosides, including flavanone glucosides, flavone glucosides, flavonol glucosides, and isoflavone glucosides as
Koichiro Kogawa et al.
Journal of plant physiology, 164(7), 886-894 (2006-08-05)
The crude malonyltransferase from the petals of Clitoria ternatea was characterized enzymatically to investigate its role on the biosynthetic pathways of anthocyanins and flavonol glycosides. In C. ternatea, a blue flower cultivars (DB) and mauve flower variety (WM) accumulate polyacylated
Heather Seidle et al.
Journal of bacteriology, 186(8), 2499-2503 (2004-04-03)
Cfa1 was overproduced in Escherichia coli and Pseudomonas syringae, and the degree of 4'-phosphopantetheinylation was determined. The malonyl-coenzyme A:acyl carrier protein transacylase (FabD) of P. syringae was overproduced and shown to catalyze malonylation of Cfa1, suggesting that FabD plays a
Christopher J Arthur et al.
ACS chemical biology, 4(8), 625-636 (2009-06-27)
Malonylation of an acyl carrier protein (ACP) by malonyl Coenzyme A-ACP transacylase (MCAT) is fundamental to bacterial fatty acid biosynthesis. Here, we report the structure of the Steptomyces coelicolor (Sc) fatty acid synthase (FAS) ACP and studies of its binding
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门