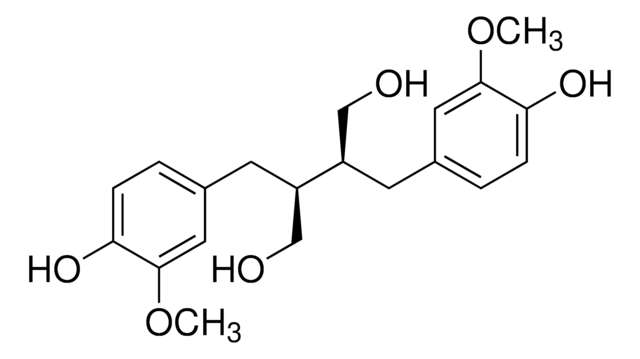
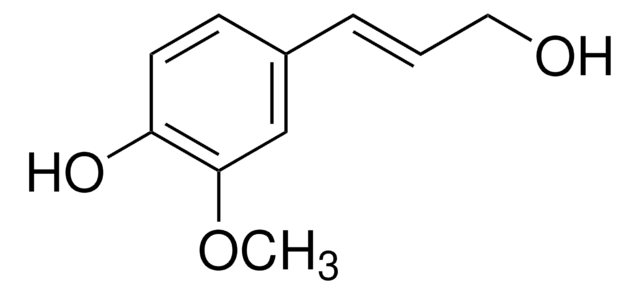
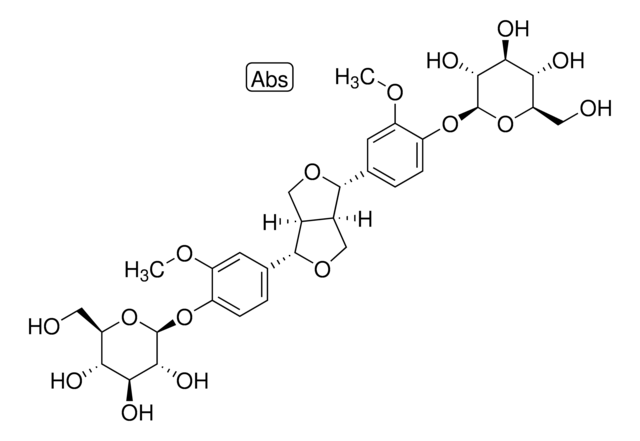
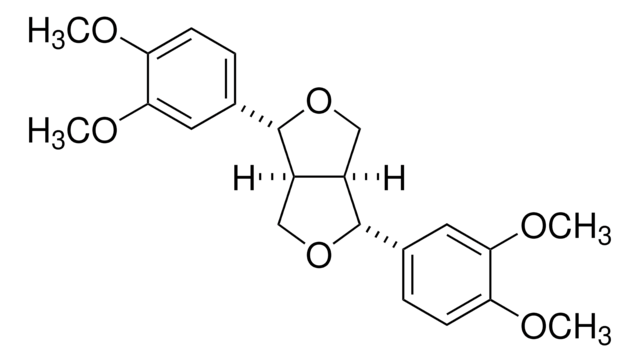
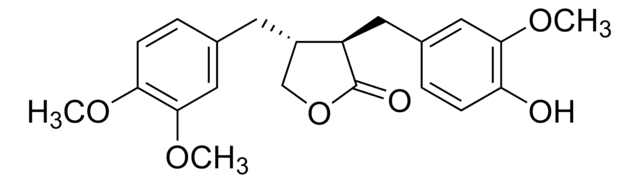
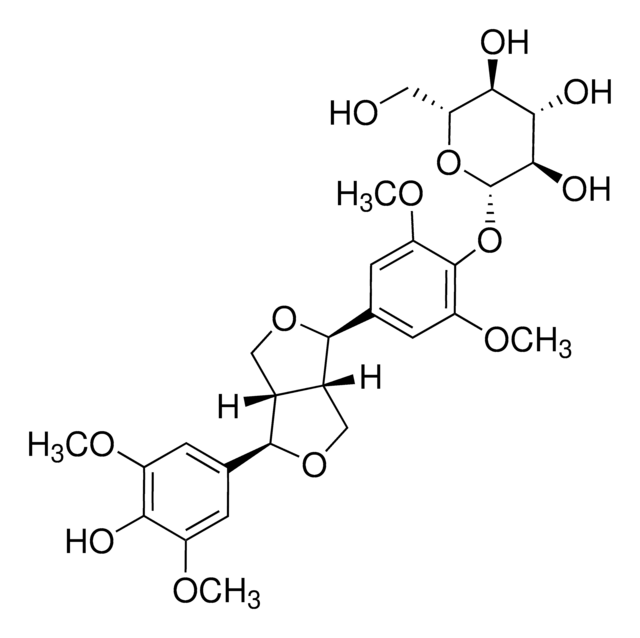
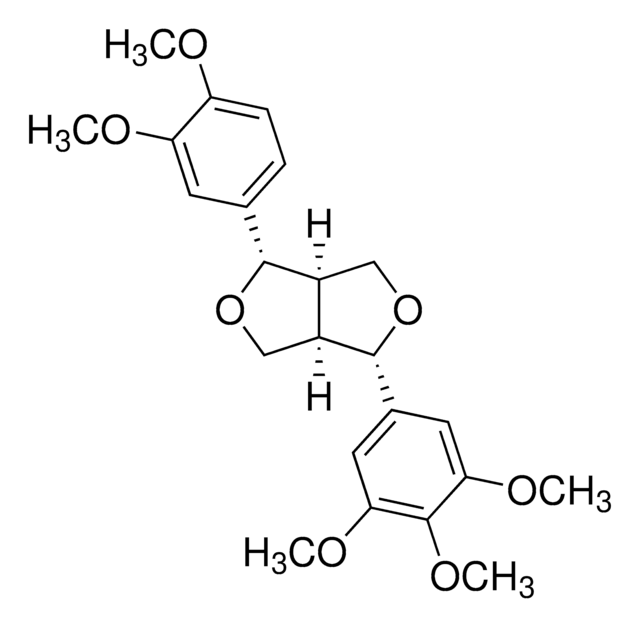
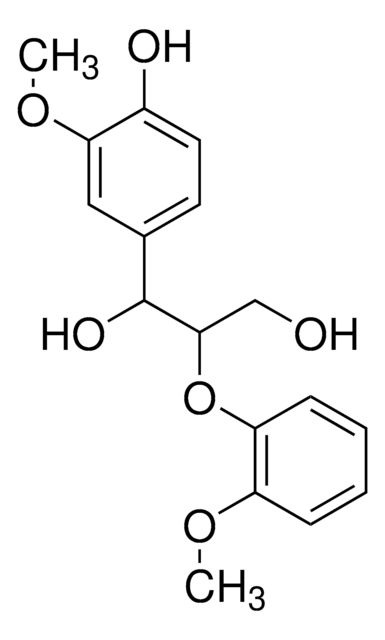
推荐产品
品質等級
化驗
≥95.0% (HPLC)
形狀
powder or crystals
應用
metabolomics
vitamins, nutraceuticals, and natural products
SMILES 字串
COc1cc(ccc1O)[C@H]2OC[C@H]3[C@@H]2CO[C@@H]3c4ccc(O)c(OC)c4
InChI
1S/C20H22O6/c1-23-17-7-11(3-5-15(17)21)19-13-9-26-20(14(13)10-25-19)12-4-6-16(22)18(8-12)24-2/h3-8,13-14,19-22H,9-10H2,1-2H3/t13-,14-,19+,20+/m0/s1
InChI 密鑰
HGXBRUKMWQGOIE-AFHBHXEDSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Pinoresinol is a secondary metabolite, a lignan found in wide varieties of plants. Structurally, it is one of the simple lignans, with a dimer of coniferyl alcohol, which forms the bicyclic ring core.
應用
Pinoresinol has been used:
- as a reference standard for qualitative and quantitative analysis of lignans from Triticale (X Triticosecale Wittmack) grains using ultra-performance liquid chromatography (UPLC) with photodiode and mass TQD detectors
- as an enterolignan precursor to study its estrogenic activity on the proliferation of human breast cancer MCF-7 cells
- as a reference standard for lignan analysis of Sesamum indicum L. seeds
生化/生理作用
松脂素存在于包括药用植物在内的多种植物中,如水生接骨木、杜仲、安息香属、连翘以及特级初榨橄榄油中。其中的接骨木属广泛分布于欧洲、亚洲和北非,并已在传统医学中作为止痛药、抗病毒药、抗炎药、止血药和利尿药用于瘀伤、骨折和水肿。松脂素可通过引起真菌质膜的损伤而显示出有效的抗真菌特性。它通过抑制TNF-α的产生(很可能是通过抑制NF- κB和AP-1)而发挥出抗氧化和抗炎的作用。
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Frank C Schroeder et al.
Proceedings of the National Academy of Sciences of the United States of America, 103(42), 15497-15501 (2006-10-13)
Pinoresinol, a lignan of wide distribution in plants, is found to occur as a minor component in the defensive secretion produced by glandular hairs of caterpillars of the cabbage butterfly, Pieris rapae. The compound or a derivative is appropriated by
Hyo Won Jung et al.
Neuroscience letters, 480(3), 215-220 (2010-07-06)
The activation of microglia plays an important role in a variety of brain disorders by the excessive production of inflammatory mediators such as nitric oxide (NO), prostaglandin E(2) (PGE(2)) and proinflammatory cytokines. We investigated here whether pinoresinol isolated from the
Yun Zhu et al.
PloS one, 12(2), e0171390-e0171390 (2017-02-06)
Mammalian lignans or enterolignans are metabolites of plant lignans, an important category of phytochemicals. Although they are known to be associated with estrogenic activity, cell signaling pathways leading to specific cell functions, and especially the differences among lignans, have not
Y Fukuhara et al.
Enzyme and microbial technology, 52(1), 38-43 (2012-12-04)
Bacterial genes for the degradation of major dilignols produced in lignifying xylem are expected to be useful tools for the structural modification of lignin in plants. For this purpose, we isolated pinZ involved in the conversion of pinoresinol from Sphingobium
Kye-Won Kim et al.
The Journal of biological chemistry, 287(41), 33957-33972 (2012-08-03)
How stereoselective monolignol-derived phenoxy radical-radical coupling reactions are differentially biochemically orchestrated in planta, whereby for example they afford (+)- and (-)-pinoresinols, respectively, is both a fascinating mechanistic and evolutionary question. In earlier work, biochemical control of (+)-pinoresinol formation had been
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门