推荐产品
等級
ACS reagent
品質等級
蒸汽密度
3.5 (vs air)
蒸汽壓力
120 mmHg ( 20 °C)
化驗
≥99.0%
形狀
liquid
自燃溫度
827 °F
包含
either BHT or hydroquinone as stabilizer
expl. lim.
1-21 %, 100 °F
雜質
≤0.0007 meq/g Titr. acid
≤0.05% Peroxide (as C6H14O2)
蒸發殘留物
≤0.01%
顏色
APHA: ≤25
折射率
n20/D 1.367 (lit.)
bp
68-69 °C (lit.)
mp
−85 °C (lit.)
密度
0.725 g/mL at 25 °C (lit.)
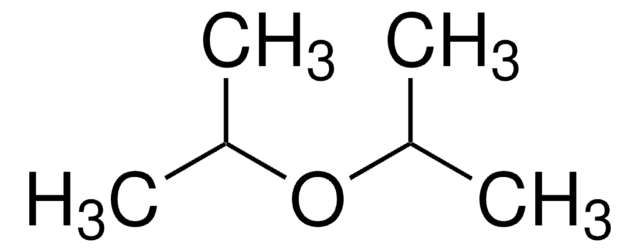
SMILES 字串
CC(C)OC(C)C
InChI
1S/C6H14O/c1-5(2)7-6(3)4/h5-6H,1-4H3
InChI 密鑰
ZAFNJMIOTHYJRJ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Diisopropyl ether (DIPE) is branched ether. It is a peroxide forming solvent. This process is catalyzed by heat, light and oxygen. For such solvents small amount of stabilizers like BHT (butylated hydroxytoluene) or hydroquinone is added which removes the free radicals that form peroxides. DIPE has been proposed as a potential alternative oxygenated fuel additive. Several methods have been proposed for its synthesis using acetone as starting material. The molecular structure and conformation has been studied using gas-phase electron diffusion. Its potential carcinogenicity has been studied in Sprague-Dawley rats.
應用
Diisopropyl ether may be used as a solvent in the following processes:
- Resolution of secondary alcohols.
- Enantioselective acylation of 1-phenethylamine.
- Synthesis of aliphatic and aromatic cyanohydrins.
訊號詞
Danger
危險聲明
危險分類
Flam. Liq. 2 - STOT SE 3
標靶器官
Central nervous system
安全危害
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
-20.2 °F - closed cup
閃點(°C)
-29 °C - closed cup
其他客户在看
Cross-linked enzyme aggregates (CLEAs): stable and recyclable biocatalysts.
Sheldon RA.
Biochemical Society Transactions, 35(6) (2007)
Molecular structure and conformation of diisopropyl ether: a gas electron diffraction investigation.
Takeuchi H, et al.
The Journal of Physical Chemistry, 91(5), 1015-1019 (1987)
The influence of reaction conditions on the photooxidation of diisopropyl ether.
Collins EM, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 176(1), 86-97 (2005)
Diisopropyl ether one-step generation from acetone-rich feedstocks.
Taylor RJ, et al.
Catalysis Letters, 68(1-2), 1-5 (2000)
Diisopropyl ether syntheses from crude acetone.
Knifton JF and D PSE.
Catalysis Letters, 57(4), 193-197 (1999)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门