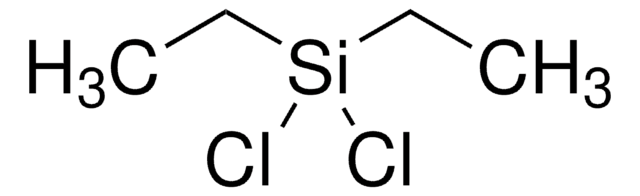
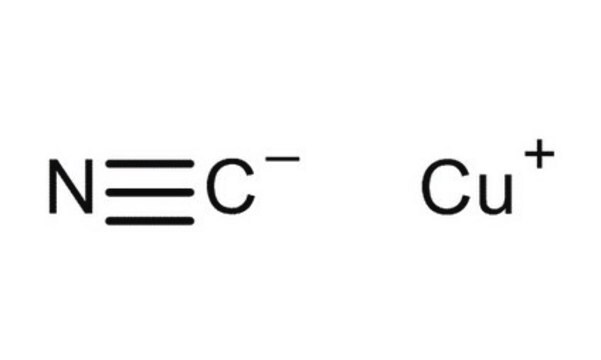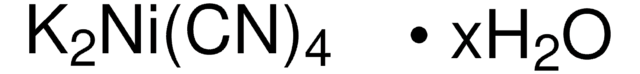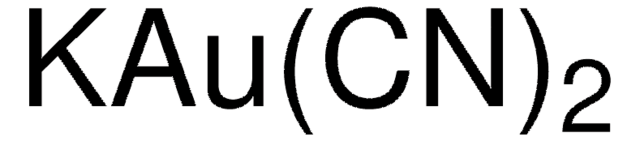推荐产品
等級
for analytical purposes
化驗
99%
形狀
powder
mp
474 °C (lit.)
密度
2.92 g/mL at 25 °C (lit.)
SMILES 字串
[Cu]C#N
InChI
1S/CN.Cu/c1-2;
InChI 密鑰
DULSAGLWMRMKCQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
应用于广泛使用的、硫代硫酸盐辅助的各种二胺-CuCN配合物合成。
訊號詞
Danger
危險分類
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2 Oral
標靶器官
Liver,spleen,Bone marrow
安全危害
儲存類別代碼
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Douglas B Grotjahn et al.
Journal of the American Chemical Society, 124(20), 5895-5901 (2002-05-16)
Copper(I) cyanide is an important reagent in organic, organometallic, and supramolecular chemistry because of both the copper center and the versatile cyanide ligand. Solid-phase CuCN and many of its derivatives show oligomeric or polymeric structures, a trait shared by other
Virender K Sharma et al.
Environmental science & technology, 39(10), 3849-3854 (2005-06-15)
Copper(Il) cyanide (Cu(CN)4(3-)) in the gold mine industry presentsthe biggest concern in cyanide management because it is much more stable than free cyanide. Cu(CN)4(3-) is highlytoxic to aquatic life; therefore, environmentally friendly techniques are required for the removal of Cu(CN)4(3-)
Fred B. Stocker et al.
Inorganic chemistry, 35(11), 3145-3153 (1996-05-22)
The syntheses and crystal structures of the first cyanide, sulfur mixed ligand copper(I) complexes are reported. The first complex of the family was discovered when (CuCN)(3)(C(6)H(12)N(4))(2) (1) (C(6)H(12)N(4) = hexamethylenetetramine) was treated with aqueous thiourea. The sulfur ligands include thiourea
Crystal Structures of a Series of Complexes Produced by Reaction of Copper(I) Cyanide with Diamines.
Fred B. Stocker et al.
Inorganic chemistry, 38(5), 984-991 (2001-10-24)
A new synthetic procedure developed recently in our laboratories has made possible the synthesis of variety of new complexes of CuCN with diamines. Synthesis was effected by adding the ligand to a solution of CuCN in aqueous sodium thiosulfate. This
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门