推荐产品
等級
pharmaceutical primary standard
API 家族
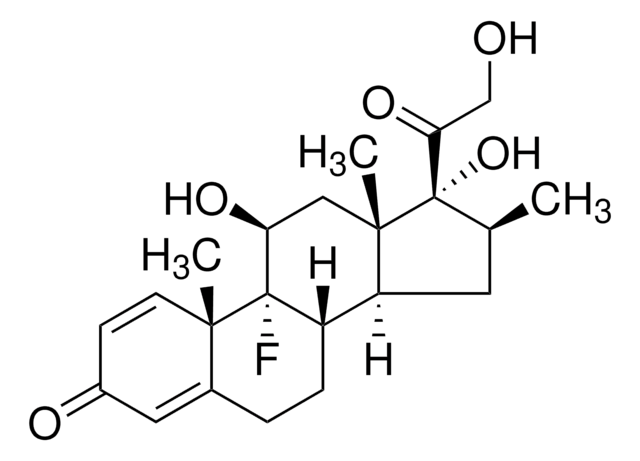
dexamethasone
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
[Na+].[Na+].[H][C@@]12CCC3=CC(=O)C=C[C@]3(C)[C@@]1(F)[C@@H](O)C[C@@]4(C)[C@@]2([H])C[C@@H](C)[C@]4(O)C(=O)COP([O-])([O-])=O
InChI
1S/C22H30FO8P.2Na/c1-12-8-16-15-5-4-13-9-14(24)6-7-19(13,2)21(15,23)17(25)10-20(16,3)22(12,27)18(26)11-31-32(28,29)30;;/h6-7,9,12,15-17,25,27H,4-5,8,10-11H2,1-3H3,(H2,28,29,30);;/q;2*+1/p-2/t12-,15+,16+,17+,19+,20+,21+,22+;;/m1../s1
InChI 密鑰
PLCQGRYPOISRTQ-FCJDYXGNSA-L
基因資訊
human ... NR3C1(2908)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Dexamethasone sodium phosphate for peak identification EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Hüseyin Simavli et al.
Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics, 30(8), 650-656 (2014-07-02)
To evaluate the inhibitory effects of propranolol, a nonselective and lipophilic β-adrenergic receptor blocker, on alkali-induced corneal neovascularization (NV). Corneal NV was induced in 24 eyes of 24 Wistar rats using NaOH. Following alkali burn, animals were randomized into 4
Soon Hyung Park et al.
The Laryngoscope, 124(6), 1444-1451 (2013-10-25)
We investigated whether the round window membrane (RWM) vibration can facilitate dexamethasone perfusion via the RWM in patients with sudden hearing loss. Prospective study. We first performed an in vitro study using a semipermeable membrane. In the subsequent in vivo
Marcus Ang et al.
The British journal of ophthalmology, 98(8), 1028-1032 (2014-03-29)
To describe outcomes and complications following Descemet's stripping automated endothelial keratoplasty (DSAEK) in eyes with pseudophakic bullous keratopathy (BK) while retaining the anterior chamber intraocular lenses (ACIOL). We included consecutive patients who underwent DSAEK for BK at a single tertiary
Saleh Sulaimana et al.
Environmental technology, 35(13-16), 1945-1955 (2014-06-25)
Stability and removal of dexamethasone sodium phosphate (DSP) from wastewater produced at Al-Quds University Campus were investigated. Kinetic studies in both pure water and wastewater coming from secondary treatment (activated sludge) demonstrated that the anti-inflammatory DSP underwent degradation to its
Yakov Goldich et al.
American journal of ophthalmology, 159(1), 155-159 (2014-12-03)
To compare objective and subjective outcomes after Descemet membrane endothelial keratoplasty (DMEK) and Descemet stripping automated endothelial keratoplasty (DSAEK) in the fellow eye of the same patients. Single-center, retrospective case series. Seventeen patients with bilateral Fuchs endothelial dystrophy who underwent
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门