Y0001062
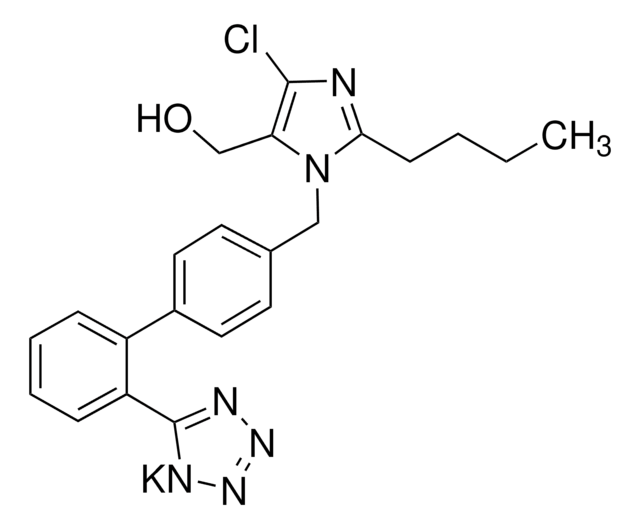
氯沙坦钾
European Pharmacopoeia (EP) Reference Standard
别名:
2-丁基-4-氯-1-[[2′-(2H-四唑-5-基)[1,1′-联苯]-4-基]甲基]- 1H-咪唑-5-甲醇钾盐, 2-丁基-4-氯-1-{[2′-(1H-四唑-5-yl)(1,1′-联苯)-4-yl]甲基}-1H-咪唑-5-甲醇 单钾盐, DuP 753, MK 954, 钾5-(4′-((2-丁基-4-氯-5-(羟甲基)-1H-咪唑-1-基)甲基)-[1,1′-联苯]-2-yl)四唑-1-ide
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C22H22ClKN6O
CAS号:
分子量:
461.00
Beilstein:
5845770
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
losartan
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
CCCCc1nc(Cl)c(CO)n1Cc2ccc(cc2)-c3ccccc3-c4nnnn4[K]
InChI
1S/C22H22ClN6O.K/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22;/h4-7,9-12,30H,2-3,8,13-14H2,1H3;/q-1;+1
InChI 密鑰
OXCMYAYHXIHQOA-UHFFFAOYSA-N
基因資訊
human ... AGTR1(185)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Losartan potassium EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
口服、强效、选择性的竞争血管紧张素II受体1型 (AT1)拮抗剂。
氯沙坦钾是一种口服、强效、选择性的竞争血管紧张素II受体1型 (AT1)拮抗剂。氯沙坦钾用于治疗高血压。
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Satoshi Terada et al.
The Journal of bone and joint surgery. American volume, 95(11), 980-988 (2013-06-20)
Muscle contusions are a common type of muscle injury and are frequently encountered in athletes and military personnel. Although these injuries are capable of healing in most instances, incomplete functional recovery often occurs because of the development of fibrosis in
Christiane Pees et al.
The American journal of cardiology, 112(9), 1477-1483 (2013-07-23)
Since 2008, when angiotensin II type I receptor blockade with losartan was introduced in the prevention of cardiovascular manifestation of Marfan syndrome (MFS), a specific treatment to address the cardiovascular lesions became available. The present study aimed to compare the
K L Goa et al.
Drugs, 51(5), 820-845 (1996-05-01)
Losartan potassium is an orally active, nonpeptide angiotensin II (AII) receptor antagonist. It is the first of a new class of drugs to be introduced for clinical use in hypertension. This novel agent binds competitively and selectively to the AII
M Burnier et al.
Journal of hypertension. Supplement : official journal of the International Society of Hypertension, 13(1), S23-S28 (1995-07-01)
The evaluation of a new drug in normotensive volunteers provides important pharmacodynamic and pharmacokinetic information as long as the compound has a specific mechanism of action which can be evaluated in healthy subjects as well as in patients. The purpose
L M Burrell
Drug safety, 16(1), 56-65 (1997-01-01)
Losartan potassium is the first of a new class of orally active antihypertensive drugs which antagonise the action of angiotensin (AT) II at the AT1 receptor subtype. Losartan potassium is converted by the liver to the active metabolite E-3174, which
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门