推荐产品
等級
pharmaceutical primary standard
API 家族
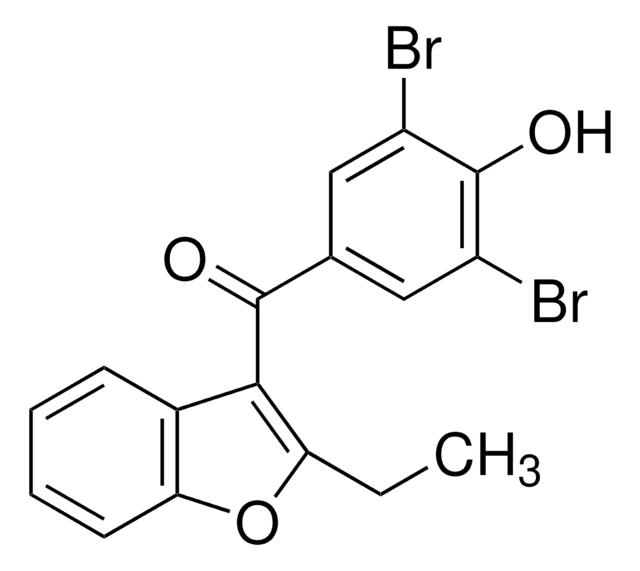
benzbromarone
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
CCc1oc2ccccc2c1C(=O)c3cc(Br)c(O)c(Br)c3
InChI
1S/C17H12Br2O3/c1-2-13-15(10-5-3-4-6-14(10)22-13)16(20)9-7-11(18)17(21)12(19)8-9/h3-8,21H,2H2,1H3
InChI 密鑰
WHQCHUCQKNIQEC-UHFFFAOYSA-N
基因資訊
human ... SLC22A12(116085)
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Benzbromarone EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Dingyu Wang et al.
Cardiovascular toxicology, 21(3), 192-205 (2020-10-26)
High levels of serum uric acid is closely associated with atrial fibrillation (AF); nonetheless, the detailed mechanisms remain unknown. Therefore, this work examined the intricate mechanisms of AF triggered by hyperuricemia and the impact of the uricosuric agent benzbromarone on
Tip W Loo et al.
Biochemistry, 50(21), 4393-4395 (2011-04-28)
Deletion of Phe508 from the first nucleotide-binding domain of the CFTR chloride channel causes cystic fibrosis because it inhibits protein folding. Indirect approaches such as incubation at low temperatures can partially rescue ΔF508 CFTR, but the protein is unstable at
Atsushi Iwamura et al.
Drug metabolism and disposition: the biological fate of chemicals, 39(5), 838-846 (2011-02-16)
Drug-induced hepatotoxicity is a major problem in drug development, and reactive metabolites generated by cytochrome P450s are suggested to be one of the causes. CYP2C9 is one of the major enzymes in hepatic drug metabolism. In the present study, we
Fernanda Cristina Mazali et al.
Nephron. Experimental nephrology, 120(1), e12-e19 (2011-12-01)
Hyperuricemia frequently complicates cyclosporine (CsA) therapy. Previous studies have shown that hyperuricemia exacerbates interstitial and vascular lesions in the cyclosporine model. We tested the hypothesis that normalization of uric acid could prevent the development of cyclosporine toxicity. CsA nephropathy was
Tip W Loo et al.
Biochemical pharmacology, 83(3), 345-354 (2011-12-06)
The most common cause of cystic fibrosis is deletion of Phe508 in the first nucleotide-binding domain (NBD) of the CFTR chloride channel, which inhibits protein folding. ΔF508 CFTR can be rescued by indirect approaches such as low temperature but the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门