推荐产品
等級
pharmaceutical primary standard
API 家族
rifamycin, rifampicin
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
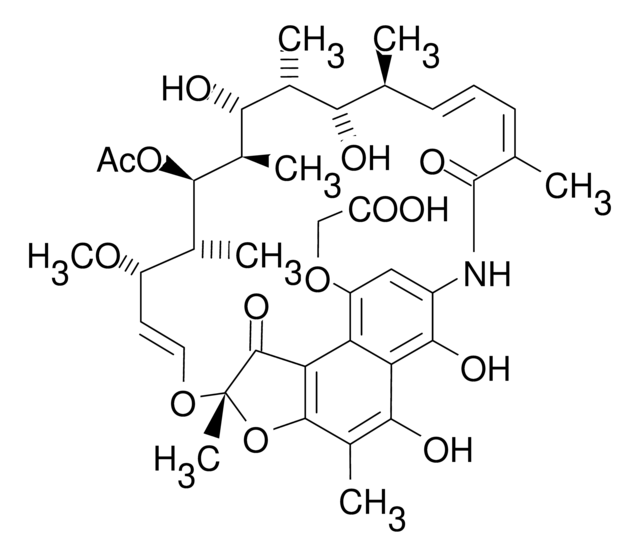
SMILES 字串
N1C2=CC(=O)c3c4c(c(c(c3C2=O)O)C)O[C@](O\C=C\[C@@H]([C@H]([C@H]([C@@H]([C@@H]([C@@H]([C@H]([C@H](\C=C\C=C(/C1=O)\C)C)O)C)O)C)OC(=O)C)C)OC)(C4=O)C
InChI
1S/C37H45NO12/c1-16-11-10-12-17(2)36(46)38-23-15-24(40)26-27(32(23)44)31(43)21(6)34-28(26)35(45)37(8,50-34)48-14-13-25(47-9)18(3)33(49-22(7)39)20(5)30(42)19(4)29(16)41/h10-16,18-20,25,29-30,33,41-43H,1-9H3,(H,38,46)/b11-10+,14-13+,17-12-/t16-,18+,19+,20+,25-,29-,30+,33+,37-/m0/s1
InChI 密鑰
BTVYFIMKUHNOBZ-ODRIEIDWSA-N
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Rifamycin S EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Aquatic Acute 1 - Aquatic Chronic 1 - STOT SE 2
標靶器官
Liver
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
Ming Chen et al.
The Journal of organic chemistry, 78(1), 3-8 (2012-06-19)
Syntheses of the C(15)-C(27) fragments of chaxamycins A/D, rifamycin S, and the C(12)-C(24) fragment of salinisporamycin have been accomplished in 10 steps from commercially available starting materials. Three crotylboron reagents were utilized to construct the seven contiguous stereocenters in these
U C Banerjee
Biomaterials, artificial cells, and immobilization biotechnology : official journal of the International Society for Artificial Cells and Immobilization Biotechnology, 21(5), 675-683 (1993-01-01)
Rifamycin oxidase of Curvularia lunata was immobilized on alginate gel. The pH and temperature optima of the immobilized enzyme preparation were 6.5 and 50 degrees C, respectively. Transformation reaction was carried out with the immobilized enzyme preparation. It took 8
Molecular structure and conformation of rifamycin S, a potent inhibitor of DNA-dependent RNA polymerase.
S K Arora et al.
The Journal of antibiotics, 45(3), 428-431 (1992-03-01)
S BouzBouz et al.
Organic letters, 3(25), 3995-3998 (2001-12-12)
[reaction: see text] An efficient, simple method has been developed for the stereocontrolled synthesis of polypropionate stereopentads in high enantio- and diastereomeric purities.
O Ghisalba et al.
The Journal of antibiotics, 35(1), 74-80 (1982-01-01)
The transformation of rifamycin S into rifamycins B and L was reinvestigated in order to establish more detailed pathways. Our results exclude rifamycin O as a common progenitor in the biosyntheses of rifamycins B and L. Rifamycins B and L
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门