推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to USP 1466674
蒸汽壓力
5 mmHg ( 20 °C)
形狀
liquid
CofA
current certificate can be downloaded
包裝
pkg of 100 mg
折射率
n20/D 1.437 (lit.)
bp
153 °C/774 mmHg (lit.)
密度
1.01 g/mL (lit.)
應用
pharmaceutical
儲存溫度
2-8°C
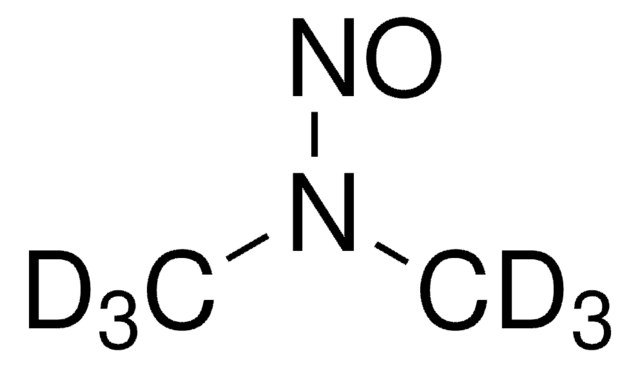
SMILES 字串
CN(C)N=O
InChI
1S/C2H6N2O/c1-4(2)3-5/h1-2H3
InChI 密鑰
UMFJAHHVKNCGLG-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. It is analyzed using GMP validated instruments as per pharmacopeia monograph methods and is traceable to Unites States Pharmacopeia (USP), European Pharmacopeia (EP), and British Pharmacopeia (BP) primary standards, wherever applicable.
It is provided with a comprehensive certificate of analysis (CoA) containing a certified purity value, calculated by the mass balance approach. All information regarding the use of this CRM can be found on the certificate of analysis.N-Nitrosodimethylamine (NDMA) is a nitrosamine that occurs as an impurity in sartan angiotensin II receptor blocker drugs.
It is provided with a comprehensive certificate of analysis (CoA) containing a certified purity value, calculated by the mass balance approach. All information regarding the use of this CRM can be found on the certificate of analysis.N-Nitrosodimethylamine (NDMA) is a nitrosamine that occurs as an impurity in sartan angiotensin II receptor blocker drugs.
應用
N-Nitrosodimethylamine CRM may also find uses as given below:
- Determination of N-Nitrosodimethylamine (NDMA) as an impurity in four valsartan APIs and tablets by high-performance liquid chromatography (HPLC)
- Quantitative analysis of NDMA in valsartan pharmaceutical formulations by capillary electrophoresis-nanospray-mass spectrometry
- Simultaneous determination of N-nitrosodimethylamine and N-nitrosomethylethylamine in drug substances and products containing sartans, ranitidine, and metformin by solid-phase extraction (SPE) and gas chromatography-tandem mass spectrometry (GC-MS/MS)
- Analysis of NDMA in the olmesartan API and tablets by high-performance liquid chromatography-mass spectrometry (HPLC-MS)
- Development and validation of an HPLC-MS/MS method for separation and quantification of NDMA impurity for quality control of ranitidine products
生化/生理作用
诱发大、小鼠出现胃癌、肝癌、肾癌和肺癌。
其他說明
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
腳註
To see an example of a Certificate of Analysis for this material enter LRAC4355 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
訊號詞
Danger
危險分類
Acute Tox. 1 Inhalation - Acute Tox. 2 Oral - Aquatic Chronic 2 - Carc. 1B - STOT RE 1
標靶器官
Liver
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
141.8 °F - closed cup
閃點(°C)
61.0 °C - closed cup
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门