PHR2021
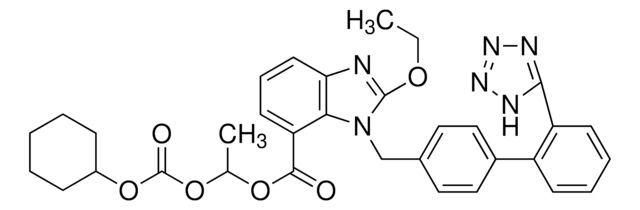
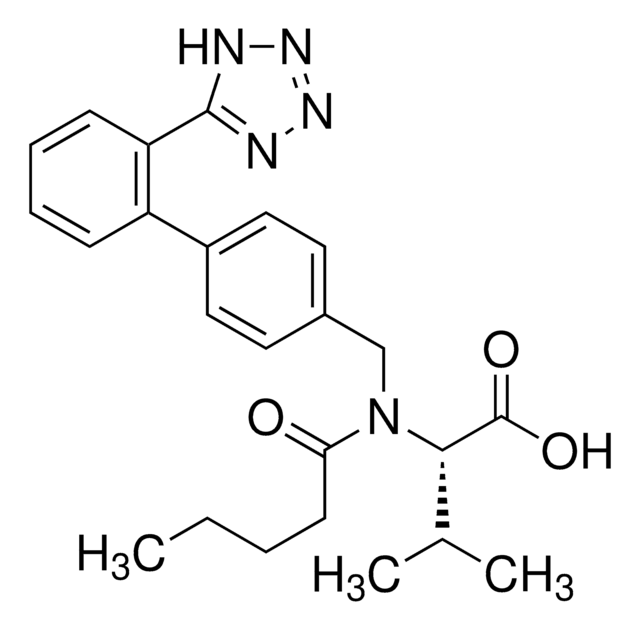
坎地沙坦酯相关化合物G
Pharmaceutical Secondary Standard; Certified Reference Material
别名:
Candesartan, 1-{[2′-(1H-Tetrazol-5-yl)biphenyl-4-yl]methyl}-2-ethoxybenzimidazole-7-carboxylic acid(Candesartan Cilexetil Related Compound G), 2-Ethoxy 1-{[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}benzimidazole-7-carboxylic acid
About This Item
推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to USP 1087870
API 家族
candesartan
CofA
current certificate can be downloaded
包裝
pkg of 20 mg
應用
pharmaceutical
形式
neat
儲存溫度
2-8°C
SMILES 字串
[nH]1nnnc1c2c(cccc2)c3ccc(cc3)C[n]4c5c(nc4OCC)cccc5C(=O)O
InChI
1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29)
InChI 密鑰
HTQMVQVXFRQIKW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
應用
分析報告
其他說明
腳註
推薦產品
相關產品
訊號詞
Warning
危險聲明
危險分類
Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门