PHR2020
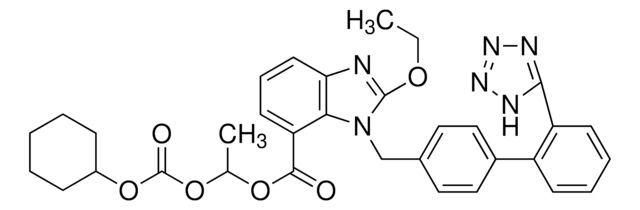
坎地沙坦酯相关化合物 F
Pharmaceutical Secondary Standard; Certified Reference Material
别名:
(1-(Cyclohexyloxycarbonyloxy]ethyl 2-ethoxy-1-{[2′-(2-ethyl-2H-tetrazol-5-yl)-biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C35H38N6O6
分子量:
638.71
分類程式碼代碼:
41116107
NACRES:
NA.24
推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to USP 1087869
API 家族
candesartan
CofA
current certificate can be downloaded
包裝
pkg of 20 mg
應用
pharmaceutical small molecule
形式
neat
儲存溫度
2-8°C
相关类别
一般說明
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Candesartan cilexetil is a selective antagonist to Angiotensin II receptors, that plays a crucial role in blood pressure regulation and sodium homeostasis, and is popularly used for the treatment of hypertension, diabetic nephropathy and congestive heart failure.
Candesartan cilexetil is a selective antagonist to Angiotensin II receptors, that plays a crucial role in blood pressure regulation and sodium homeostasis, and is popularly used for the treatment of hypertension, diabetic nephropathy and congestive heart failure.
應用
Candesartan cilexetil may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by spectrophotometric and chromatographic techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAB4750 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
相關產品
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
A comparison of the efficacy and duration of action of candesartan cilexetil and losartan as assessed by clinic and ambulatory blood pressure after a missed dose, in truly hypertensive patients: a placebo-controlled, forced titration study
Lacourciere Y and Asmar R
American Journal of Hypertension, 12(12), 1181-1187 (1999)
Determination of candesartan cilexetil in tablets by spectrofluorimetry
Sakur AA and Hanan F
International Journal of Pharmaceutical Sciences Review and Research, 4(2), 60-63 (2010)
Reduction of bleomycin induced lung fibrosis by candesartan cilexetil, an angiotensin II type 1 receptor antagonist
Otsuka M, et al.
Thorax, 59(1), 31-38 (2004)
Characterization of conjugated metabolites of a new angiotensin II receptor antagonist, candesartan cilexetil, in rats by liquid chromatography/electrospray tandem mass spectrometry following chemical derivatization
Kondo T, et al.
Journal of Mass Spectrometry : Jms, 31(8), 873-878 (1996)
Q-Analysis Spectrophotometric methods for estimation of Candesartan Cilexetil and Hydrochlorothiazide in tablet dosage form
Jignesh P, et al.
Journal of Chemical and Pharmaceutical Research, 2(3), 10-14 (2010)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门