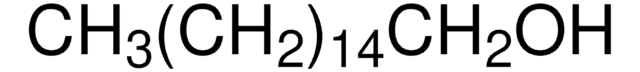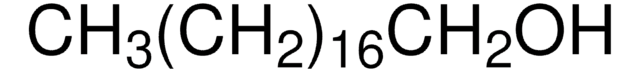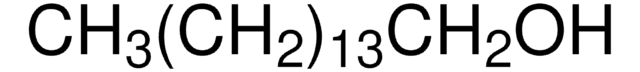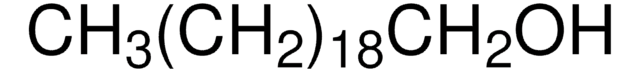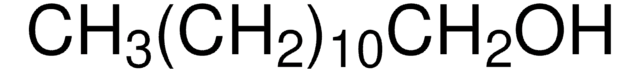推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. C0990000
traceable to USP 1103003
蒸汽密度
8.34 (vs air)
蒸汽壓力
<0.01 mmHg ( 43 °C)
API 家族
cetyl alcohol
CofA
current certificate can be downloaded
自燃溫度
483 °F
expl. lim.
8 %
技術
HPLC: suitable
gas chromatography (GC): suitable
bp
179-181 °C/10 mmHg (lit.)
mp
48-50 °C (lit.)
密度
0.818 g/mL at 25 °C (lit.)
應用
cleaning products
cosmetics
food and beverages
personal care
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-30°C
SMILES 字串
CCCCCCCCCCCCCCCCO
InChI
1S/C16H34O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17/h17H,2-16H2,1H3
InChI 密鑰
BXWNKGSJHAJOGX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
應用
Cetyl Alcohol may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by various chromatography techniques.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析報告
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
其他說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
腳註
To see an example of a Certificate of Analysis for this material enter LRAA7153 in the slot below. This is an example certificate only and may not be the lot that you receive.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
nwg
閃點(°F)
338.0 °F
閃點(°C)
170 °C
其他客户在看
The application of chromatography to the analysis of pharmaceutical creams.
Van de Vaart FJ, et al.
Chromatographia, 16(1), 247-250 (1982)
HPLC-fluorescence determination of chlorocresol and chloroxylenol in pharmaceuticals.
Gatti R, et al.
Journal of Pharmaceutical and Biomedical Analysis, 16(3), 405-412 (1997)
N Dashti et al.
Chemosphere, 70(3), 475-479 (2007-08-07)
Bacteria and fungi in pristine and oily desert soil samples were counted on inorganic medium aliquots containing 0.5% hexadecane, hexadecanol, hexadecanal or hexadecanoic acid, as sole sources of carbon and energy. It was found that the carbon and energy source
Eddy Castellanos Gil et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 69(1), 303-311 (2007-12-07)
A novel oral controlled delivery system for propranolol hydrochloride (PPL) was developed and optimized using wet granulation process. We are studying the ability of subcoating with Kollidon VA 64 as a barrier to water penetration in matrix cores combined hydrophilic
Daniel Wehrung et al.
Colloids and surfaces. B, Biointerfaces, 94, 259-265 (2012-03-06)
The main objective of the study is to investigate the efficacy of Gelucire 44/14 (gelucire) in facilitating formation of cetyl alcohol (CA)-based nanoparticle (NP) and to assess the effects on key NP properties and functions. NPs from oil-in-water nanoemulsion precursors
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门