推荐产品
等級
pharmaceutical primary standard
API 家族
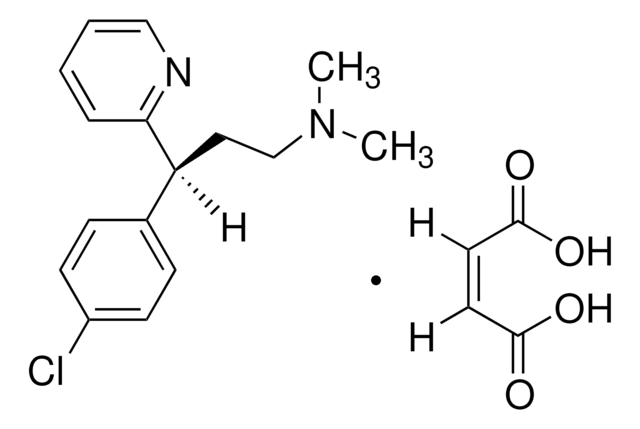
dexchlorpheniramine
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
[H]\C(=C(/[H])C(O)=O)C(O)=O.CN(C)CC[C@@H](c1ccc(Cl)cc1)c2ccccn2
InChI
1S/C16H19ClN2.C4H4O4/c1-19(2)12-10-15(16-5-3-4-11-18-16)13-6-8-14(17)9-7-13;5-3(6)1-2-4(7)8/h3-9,11,15H,10,12H2,1-2H3;1-2H,(H,5,6)(H,7,8)/b;2-1-/t15-;/m0./s1
InChI 密鑰
DBAKFASWICGISY-DASCVMRKSA-N
基因資訊
human ... HRH1(3269)
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Dexchlorpheniramine maleate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
O Cáceres Calle et al.
Allergologia et immunopathologia, 32(5), 306-309 (2004-10-01)
Dexchlorpheniramine (DH) is a classical or first generation antihistamine belonging to the ethanolamine group. Adverse effects related to these antihistamines are frequent, but the hypersensitivity reactions described in the literature since 1940 are exceptional. We report the case of a
Anaphylaxis induced by Anisakis.
J Antón et al.
Allergologia et immunopathologia, 36(1), 53-55 (2008-02-12)
Nilton S Viana et al.
Farmaco (Societa chimica italiana : 1989), 60(11-12), 900-905 (2005-10-18)
Formulation excipients can frequently affect the drug analysis in pharmaceuticals yielding background interference by ultraviolet spectrophotometry. Sample separation procedures to diminish such interferences are usually recommended as sample pre-treatment, however it can be difficult to eliminate them and they can
José R Villada et al.
Journal of cataract and refractive surgery, 31(3), 620-621 (2005-04-07)
We report a severe anaphylactic reaction that occurred about 5 minutes after 1.0 mg of cefuroxime was injected into the anterior chamber after routine phacoemulsification and intraocular lens implantation. The patient was known to be allergic to penicillin. Immediate action
Eef L Theunissen et al.
British journal of clinical pharmacology, 61(1), 79-86 (2006-01-05)
Previous studies have demonstrated that the antihistamines mequitazine, cetirizine and dexchlorpheniramine produce mild sedation after single doses. It is unknown, however, whether acute sedation persists after repeated dosing. Therefore, this study assessed the effects of repeated dosing of these antihistamines
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门